Human Cytomegalovirus Immediate Early 86-kDa Protein Blocks Transcription and Induces Degradation of the Immature Interleukin-1β Protein during Virion-Mediated Activation of the AIM2 Inflammasome
- PMID: 30755509
- PMCID: PMC6372796
- DOI: 10.1128/mBio.02510-18
Human Cytomegalovirus Immediate Early 86-kDa Protein Blocks Transcription and Induces Degradation of the Immature Interleukin-1β Protein during Virion-Mediated Activation of the AIM2 Inflammasome
Abstract
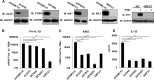
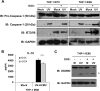
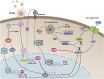
Secretion of interleukin-1β (IL-1β) represents a fundamental innate immune response to microbial infection that, at the molecular level, occurs following activation of proteolytic caspases that cleave the immature protein into a secretable form. Human cytomegalovirus (HCMV) is the archetypal betaherpesvirus that is invariably capable of lifelong infection through the activity of numerous virally encoded immune evasion phenotypes. Innate immune pathways responsive to cytoplasmic double-stranded DNA (dsDNA) are known to be activated in response to contact between HCMV and host cells. Here, we used clustered regularly interspaced short palindromic repeat (CRISPR)-CRISPR-associated protein 9 (Cas9) genome editing to demonstrate that the dsDNA receptor
Keywords: AIM2; DNA sensor; HCMV; IE2; IE86; STING; cGAS; cytomegalovirus; inflammasome; innate immunity; interferons.
Copyright © 2019 Botto et al.
Figures






Similar articles
-
Interaction between HCMV pUL83 and human AIM2 disrupts the activation of the AIM2 inflammasome.Virol J. 2017 Feb 20;14(1):34. doi: 10.1186/s12985-016-0673-5. Virol J. 2017. PMID: 28219398 Free PMC article.
-
Human cytomegalovirus immediate-early 2 protein IE86 blocks virus-induced chemokine expression.J Virol. 2006 Jan;80(2):920-8. doi: 10.1128/JVI.80.2.920-928.2006. J Virol. 2006. PMID: 16378994 Free PMC article.
-
Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection.Virology. 2000 Aug 15;274(1):39-55. doi: 10.1006/viro.2000.0448. Virology. 2000. PMID: 10936087
-
Activation and evasion of inflammasomes during viral and microbial infection.Cell Mol Life Sci. 2025 Jan 21;82(1):56. doi: 10.1007/s00018-025-05575-2. Cell Mol Life Sci. 2025. PMID: 39833559 Free PMC article. Review.
-
Modulation of the innate immune response by human cytomegalovirus.Infect Genet Evol. 2018 Oct;64:105-114. doi: 10.1016/j.meegid.2018.06.025. Epub 2018 Jun 20. Infect Genet Evol. 2018. PMID: 29935337 Review.
Cited by
-
Role of AIM2 inflammasome in inflammatory diseases, cancer and infection.Eur J Immunol. 2019 Nov;49(11):1998-2011. doi: 10.1002/eji.201848070. Epub 2019 Aug 14. Eur J Immunol. 2019. PMID: 31372985 Free PMC article. Review.
-
The innate and T-cell mediated immune response during acute and chronic gammaherpesvirus infection.Front Cell Infect Microbiol. 2023 Mar 31;13:1146381. doi: 10.3389/fcimb.2023.1146381. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37065193 Free PMC article. Review.
-
The Trinity of cGAS, TLR9, and ALRs Guardians of the Cellular Galaxy Against Host-Derived Self-DNA.Front Immunol. 2021 Feb 11;11:624597. doi: 10.3389/fimmu.2020.624597. eCollection 2020. Front Immunol. 2021. PMID: 33643304 Free PMC article. Review.
-
Cytosolic DNA Sensors and CNS Responses to Viral Pathogens.Front Cell Infect Microbiol. 2020 Sep 16;10:576263. doi: 10.3389/fcimb.2020.576263. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33042875 Free PMC article. Review.
-
Herpes simplex virus type 1 inflammasome activation in proinflammatory human macrophages is dependent on NLRP3, ASC, and caspase-1.PLoS One. 2020 Feb 26;15(2):e0229570. doi: 10.1371/journal.pone.0229570. eCollection 2020. PLoS One. 2020. PMID: 32101570 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

