Two KTR Mannosyltransferases Are Responsible for the Biosynthesis of Cell Wall Mannans and Control Polarized Growth in Aspergillus fumigatus
- PMID: 30755510
- PMCID: PMC6372797
- DOI: 10.1128/mBio.02647-18
Two KTR Mannosyltransferases Are Responsible for the Biosynthesis of Cell Wall Mannans and Control Polarized Growth in Aspergillus fumigatus
Abstract
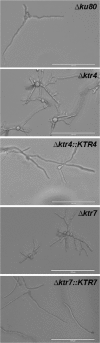
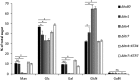
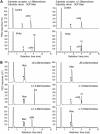
Fungal cell wall mannans are complex carbohydrate polysaccharides with different structures in yeasts and molds. In contrast to yeasts, their biosynthetic pathway has been poorly investigated in filamentous fungi. In Aspergillus fumigatus, the major mannan structure is a galactomannan that is cross-linked to the β-1,3-glucan-chitin cell wall core. This polymer is composed of a linear mannan with a repeating unit composed of four α1,6-linked and α1,2-linked mannoses with side chains of galactofuran. Despite its use as a biomarker to diagnose invasive aspergillosis, its biosynthesis and biological function were unknown. Here, we have investigated the function of three members of the Ktr (also named Kre2/Mnt1) family (Ktr1, Ktr4, and Ktr7) in A. fumigatus and show that two of them are required for the biosynthesis of galactomannan. In particular, we describe a newly discovered form of α-1,2-mannosyltransferase activity encoded by the KTR4 gene. Biochemical analyses showed that deletion of the KTR4 gene or the KTR7 gene leads to the absence of cell wall galactomannan. In comparison to parental strains, the Δktr4 and Δktr7 mutants showed a severe growth phenotype with defects in polarized growth and in conidiation, marked alteration of the conidial viability, and reduced virulence in a mouse model of invasive aspergillosis. In yeast, the KTR proteins are involved in protein 0- and N-glycosylation. This study provided another confirmation that orthologous genes can code for proteins that have very different biological functions in yeasts and filamentous fungi. Moreover, in A. fumigatus, cell wall mannans are as important structurally as β-glucans and chitin.IMPORTANCE The fungal cell wall is a complex and dynamic entity essential for the development of fungi. It allows fungal pathogens to survive environmental challenge posed by nutrient stress and host defenses, and it also is central to polarized growth. The cell wall is mainly composed of polysaccharides organized in a three-dimensional network. Aspergillus fumigatus produces a cell wall galactomannan whose biosynthetic pathway and biological functions remain poorly defined. Here, we described two new mannosyltransferases essential to the synthesis of the cell wall galactomannan. Their absence leads to a growth defect with misregulation of polarization and altered conidiation, with conidia which are bigger and more permeable than the conidia of the parental strain. This study showed that in spite of its low concentration in the cell wall, this polysaccharide is absolutely required for cell wall stability, for apical growth, and for the full virulence of A. fumigatus.
Keywords: Aspergillus fumigatus; KTR; biosynthesis; cell wall; galactomannan; mannosyltransferase.
Copyright © 2019 Henry et al.
Figures

Similar articles
-
Biosynthesis of cell wall mannan in the conidium and the mycelium of Aspergillus fumigatus.Cell Microbiol. 2016 Dec;18(12):1881-1891. doi: 10.1111/cmi.12665. Epub 2016 Oct 11. Cell Microbiol. 2016. PMID: 27603677
-
Structural basis for the core-mannan biosynthesis of cell wall fungal-type galactomannan in Aspergillus fumigatus.J Biol Chem. 2020 Nov 6;295(45):15407-15417. doi: 10.1074/jbc.RA120.013742. Epub 2020 Sep 1. J Biol Chem. 2020. PMID: 32873705 Free PMC article.
-
Identification of an α-(1→6)-Mannosyltransferase Contributing To Biosynthesis of the Fungal-Type Galactomannan α-Core-Mannan Structure in Aspergillus fumigatus.mSphere. 2022 Dec 21;7(6):e0048422. doi: 10.1128/msphere.00484-22. Epub 2022 Nov 29. mSphere. 2022. PMID: 36445154 Free PMC article.
-
Specific molecular features in the organization and biosynthesis of the cell wall of Aspergillus fumigatus.Med Mycol. 2005 May;43 Suppl 1:S15-22. doi: 10.1080/13693780400029155. Med Mycol. 2005. PMID: 16110787 Review.
-
Genes and molecules involved in Aspergillus fumigatus virulence.Rev Iberoam Micol. 2005 Mar;22(1):1-23. doi: 10.1016/s1130-1406(05)70001-2. Rev Iberoam Micol. 2005. PMID: 15813678 Review.
Cited by
-
Galactomannan Produced by Aspergillus fumigatus: An Update on the Structure, Biosynthesis and Biological Functions of an Emblematic Fungal Biomarker.J Fungi (Basel). 2020 Nov 12;6(4):283. doi: 10.3390/jof6040283. J Fungi (Basel). 2020. PMID: 33198419 Free PMC article. Review.
-
The C-Type Lectin Receptor Dectin-2 Is a Receptor for Aspergillus fumigatus Galactomannan.mBio. 2023 Feb 28;14(1):e0318422. doi: 10.1128/mbio.03184-22. Epub 2023 Jan 4. mBio. 2023. PMID: 36598192 Free PMC article.
-
The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species.Front Microbiol. 2020 Jan 9;10:2993. doi: 10.3389/fmicb.2019.02993. eCollection 2019. Front Microbiol. 2020. PMID: 31993032 Free PMC article. Review.
-
A molecular vision of fungal cell wall organization by functional genomics and solid-state NMR.Nat Commun. 2021 Nov 3;12(1):6346. doi: 10.1038/s41467-021-26749-z. Nat Commun. 2021. PMID: 34732740 Free PMC article.
-
Discovery of α-(1→6)-linked mannan structures resembling yeast N-glycan outer chains in Aspergillus fumigatus mycelium.mSphere. 2024 May 29;9(5):e0010024. doi: 10.1128/msphere.00100-24. Epub 2024 Apr 23. mSphere. 2024. PMID: 38651868 Free PMC article.
References
-
- Mouyna I, Kniemeyer O, Jank T, Loussert C, Mellado E, Aimanianda V, Beauvais A, Wartenberg D, Sarfati J, Bayry J, Prévost M-C, Brakhage AA, Strahl S, Huerre M, Latgé J-P. 2010. Members of protein O-mannosyltransferase family in Aspergillus fumigatus differentially affect growth, morphogenesis and viability. Mol Microbiol 76:1205–1221. doi:10.1111/j.1365-2958.2010.07164.x. - DOI - PubMed
-
- Wagener J, Echtenacher B, Rohde M, Kotz A, Krappmann S, Heesemann J, Ebel F. 2008. The putative α-1,2-mannosyltransferase AfMnt1 of the opportunistic fungal pathogen Aspergillus fumigatus is required for cell wall stability and full virulence. Eukaryot Cell 7:1661–1673. doi:10.1128/EC.00221-08. - DOI - PMC - PubMed
-
- Hall RA, Bates S, Lenardon MD, MacCallum DM, Wagener J, Lowman DW, Kruppa MD, Williams DL, Odds FC, Brown AJP, Gow NAR. 2013. The Mnn2 mannosyltransferase family modulates mannoprotein fibril length, immune recognition and virulence of Candida albicans. PLoS Pathog 9:e1003276. doi:10.1371/journal.ppat.1003276. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

