Dynamically stiffened matrix promotes malignant transformation of mammary epithelial cells via collective mechanical signaling
- PMID: 30755531
- PMCID: PMC6397509
- DOI: 10.1073/pnas.1814204116
Dynamically stiffened matrix promotes malignant transformation of mammary epithelial cells via collective mechanical signaling
Abstract
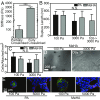
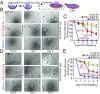
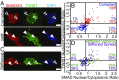
Breast cancer development is associated with increasing tissue stiffness over years. To more accurately mimic the onset of gradual matrix stiffening, which is not feasible with conventional static hydrogels, mammary epithelial cells (MECs) were cultured on methacrylated hyaluronic acid hydrogels whose stiffness can be dynamically modulated from "normal" (<150 Pascals) to "malignant" (>3,000 Pascals) via two-stage polymerization. MECs form and remain as spheroids, but begin to lose epithelial characteristics and gain mesenchymal morphology upon matrix stiffening. However, both the degree of matrix stiffening and culture time before stiffening play important roles in regulating this conversion as, in both cases, a subset of mammary spheroids remained insensitive to local matrix stiffness. This conversion depended neither on colony size nor cell density, and MECs did not exhibit "memory" of prior niche when serially cultured through cycles of compliant and stiff matrices. Instead, the transcription factor Twist1, transforming growth factor β (TGFβ), and YAP activation appeared to modulate stiffness-mediated signaling; when stiffness-mediated signals were blocked, collective MEC phenotypes were reduced in favor of single MECs migrating away from spheroids. These data indicate a more complex interplay of time-dependent stiffness signaling, spheroid structure, and soluble cues that regulates MEC plasticity than suggested by previous models.
Keywords: epithelial-to-mesenchymal transition; hyaluronic acid; hydrogel; mammary.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

