Stage-dependent cardiac regeneration in Xenopus is regulated by thyroid hormone availability
- PMID: 30755533
- PMCID: PMC6397552
- DOI: 10.1073/pnas.1803794116
Stage-dependent cardiac regeneration in Xenopus is regulated by thyroid hormone availability
Abstract
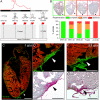
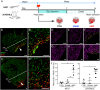
Despite therapeutic advances, heart failure is the major cause of morbidity and mortality worldwide, but why cardiac regenerative capacity is lost in adult humans remains an enigma. Cardiac regenerative capacity widely varies across vertebrates. Zebrafish and newt hearts regenerate throughout life. In mice, this ability is lost in the first postnatal week, a period physiologically similar to thyroid hormone (TH)-regulated metamorphosis in anuran amphibians. We thus assessed heart regeneration in Xenopus laevis before, during, and after TH-dependent metamorphosis. We found that tadpoles display efficient cardiac regeneration, but this capacity is abrogated during the metamorphic larval-to-adult switch. Therefore, we examined the consequence of TH excess and deprivation on the efficiently regenerating tadpole heart. We found that either acute TH treatment or blocking TH production before resection significantly but differentially altered gene expression and kinetics of extracellular matrix components deposition, and negatively impacted myocardial wall closure, both resulting in an impeded regenerative process. However, neither treatment significantly influenced DNA synthesis or mitosis in cardiac tissue after amputation. Overall, our data highlight an unexplored role of TH availability in modulating the cardiac regenerative outcome, and present X. laevis as an alternative model to decipher the developmental switches underlying stage-dependent constraint on cardiac regeneration.
Keywords: Xenopus; cardiac regeneration; extracellular matrix; metamorphosis; thyroid hormone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- World Health Organisation 2018 The top 10 causes of death: Fact sheet. Available at https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of.... Accessed February 1, 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

