Immunoglobulin G modulation of the melanocortin 4 receptor signaling in obesity and eating disorders
- PMID: 30755592
- PMCID: PMC6372612
- DOI: 10.1038/s41398-019-0422-9
Immunoglobulin G modulation of the melanocortin 4 receptor signaling in obesity and eating disorders
Abstract
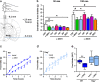
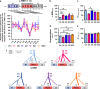
Melanocortin 4 receptor (MC4R) plays a key role in regulation of appetite activated by its main ligand α-melanocyte-stimulating hormone (α-MSH) in both central and peripheral targets. α-MSH also binds to circulating immunoglobulins (Igs) but the functional significance of such immune complexes (ICs) in MC4R signaling in normal and pathological conditions of altered appetite has remained unknown. To address this question, we analyzed plasma levels, affinity kinetics, and binding epitopes of α-MSH-reactive IgG extracted from plasma samples of female patients with hyperphagic obesity, anorexia nervosa, bulimia nervosa, binge-eating disorder, and healthy controls. Ability of α-MSH/IgG IC to bind and activate human MC4R were studied in vitro and to influence feeding behavior in vivo in rodents. We found that α-MSH-reactive IgG were low in obese but increased in anorectic and bulimic patients and displayed different epitope and kinetics of IC formation. Importantly, while α-MSH/IgG IC from all subjects were binding and activating MC4R, the receptor binding affinity was decreased in obesity. Additionally, α-MSH/IgG IC had lower MC4R-mediated cAMP activation threshold as compared with α-MSH alone in all but not obese subjects. Furthermore, the cellular internalization rate of α-MSH/IgG IC by MC4R-expressing cells was decreased in obese but increased in patients with anorexia nervosa. Moreover, IgG from obese patients prevented central anorexigenic effect of α-MSH. These findings reveal that MC4R is physiologically activated by IC formed by α-MSH/IgG and that different levels and molecular properties of α-MSH-reactive IgG underlie biological activity of such IC relevant to altered appetite in obesity and eating disorders.
Conflict of interest statement
S.O.F. is a co-founder and consultant of TargEDys, SA. N.L. and R.L. were employees of TargEDys, SA. P.D. is a co-founder of TargEDys, SA and a member of its board and has received research grants from Nestlé and Fresenius Kabi and honoraria for speeches and consulting from Nestlé, Fresenius-Kabi, and Aguettant. The other authors declare that they have no conflict of interest.
Figures




Similar articles
-
Effects of rabbit anti-α-melanocyte-stimulating hormone (α-MSH) immunoglobulins on α-MSH signaling related to food intake control.Neuropeptides. 2014 Feb;48(1):21-7. doi: 10.1016/j.npep.2013.10.017. Epub 2013 Nov 1. Neuropeptides. 2014. PMID: 24238616
-
Bacterial ClpB heat-shock protein, an antigen-mimetic of the anorexigenic peptide α-MSH, at the origin of eating disorders.Transl Psychiatry. 2014 Oct 7;4(10):e458. doi: 10.1038/tp.2014.98. Transl Psychiatry. 2014. PMID: 25290265 Free PMC article.
-
Sex-related effects of nutritional supplementation of Escherichia coli: relevance to eating disorders.Nutrition. 2015 Mar;31(3):498-507. doi: 10.1016/j.nut.2014.11.003. Epub 2014 Dec 5. Nutrition. 2015. PMID: 25701341
-
Structural Complexity and Plasticity of Signaling Regulation at the Melanocortin-4 Receptor.Int J Mol Sci. 2020 Aug 10;21(16):5728. doi: 10.3390/ijms21165728. Int J Mol Sci. 2020. PMID: 32785054 Free PMC article. Review.
-
On the origin of eating disorders: altered signaling between gut microbiota, adaptive immunity and the brain melanocortin system regulating feeding behavior.Curr Opin Pharmacol. 2019 Oct;48:82-91. doi: 10.1016/j.coph.2019.07.004. Epub 2019 Aug 17. Curr Opin Pharmacol. 2019. PMID: 31430598 Review.
Cited by
-
Shedding light on biological sex differences and microbiota-gut-brain axis: a comprehensive review of its roles in neuropsychiatric disorders.Biol Sex Differ. 2022 Mar 25;13(1):12. doi: 10.1186/s13293-022-00422-6. Biol Sex Differ. 2022. PMID: 35337376 Free PMC article. Review.
-
Plasma Peptide Concentrations and Peptide-Reactive Immunoglobulins in Patients with Eating Disorders at Inclusion in the French EDILS Cohort (Eating Disorders Inventory and Longitudinal Survey).Nutrients. 2020 Feb 18;12(2):522. doi: 10.3390/nu12020522. Nutrients. 2020. PMID: 32085628 Free PMC article.
-
Host Starvation and Female Sex Influence Enterobacterial ClpB Production: A Possible Link to the Etiology of Eating Disorders.Microorganisms. 2020 Apr 7;8(4):530. doi: 10.3390/microorganisms8040530. Microorganisms. 2020. PMID: 32272706 Free PMC article.
-
Current Aspects of the Role of Autoantibodies Directed Against Appetite-Regulating Hormones and the Gut Microbiome in Eating Disorders.Front Endocrinol (Lausanne). 2021 Apr 19;12:613983. doi: 10.3389/fendo.2021.613983. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33953692 Free PMC article. Review.
-
An evidence review of the association of immune and inflammatory markers with obesity-related eating behaviors.Front Immunol. 2022 Jul 15;13:902114. doi: 10.3389/fimmu.2022.902114. eCollection 2022. Front Immunol. 2022. PMID: 35911732 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

