Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival by inducing HIF-1α in injured neuronal cells derived exosomes culture system
- PMID: 30755595
- PMCID: PMC6372680
- DOI: 10.1038/s41419-019-1410-y
Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival by inducing HIF-1α in injured neuronal cells derived exosomes culture system
Abstract
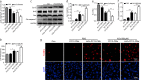
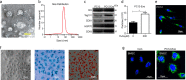
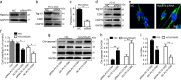
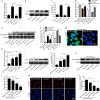
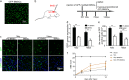
Bone marrow derived stem cells (BMSCs) transplantation are viewed as a promising therapeutic candidate for spinal cord injury (SCI). However, the inflammatory microenvironment in the spinal cord following SCI limits the survival and efficacy of transplanted BMSCs. In this study, we investigate whether injured neuronal cells derived exosomes would influence the survival of transplanted BMSCs after SCI. In order to mimic the microenvironment in SCI that the neuronal cells or transplanted BMSCs suffer in vivo, PC12 cells conditioned medium and PC12 cell's exosomes collected from H2O2-treated PC12 cell's culture medium were cultured with BMSCs under oxidative stress in vitro. PC12 cells conditioned medium and PC12 cell's exosomes significantly accelerated the apoptosis of BMSCs induced by H2O2. Moreover, the cleaved caspase-3, cytochrome (Cyt) C, lactate dehydrogenase (LDH) releases, and apoptotic percentage were increased, and the ratio of Bcl-2/Bax and cell viability were decreased. Inhibition of exosome secretion via Rab27a small interfering RNA prevented BMSCs apoptosis in vitro. In addition, hypoxia-preconditioned promoted the survival of BMSCs under oxidative stress both in vivo after SCI and in vitro. Our results also indicate that HIF-1α plays a central role in the survival of BMSCs in hypoxia pretreatment under oxidative stress conditions. siRNA-HIF-1α increased apoptosis of BMSCs; in contrast, HIF-1α inducer FG-4592 attenuated apoptosis of BMSCs. Taken together, we found that the injured PC12 cells derived exosomes accelerate BMSCs apoptosis after SCI and in vitro, hypoxia pretreatment or activating expression of HIF-1α to be important in the survival of BMSCs after transplantation, which provides a foundation for application of BMSCs in therapeutic potential for SCI.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- S. R. Andresen F, et al. Finnerup, pain, spasticity and quality of life in individuals with traumatic spinal cord injury in Denmark. Spinal Cord. 2016;54:973–979. - PubMed
-
- C. S. Rivers N, et al. Health conditions: effect on function, health-related quality of life, and life satisfaction after traumatic spinal cord injury. a prospective observational registry cohort study. Arch. Phys. Med. Rehabil. 2018;99:443–451. - PubMed
-
- S. V. Hiremath NS, et al. Longitudinal prediction of quality-of-life scores and locomotion in individuals with traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 2017;98:2385–2392. - PubMed
-
- Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4:451–464. - PubMed
-
- Tykocki T, Poniatowski L, Czyz M, Koziara M, Wynne-Jones G. Intraspinal pressure monitoring and extensive duroplasty in the acute phase of traumatic spinal cord injury: a systematic review. World Neurosurg. 2017;105:145–152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

