Direct dehydrogenative alkyl Heck-couplings of vinylarenes with umpolung aldehydes catalyzed by nickel
- PMID: 30755610
- PMCID: PMC6372677
- DOI: 10.1038/s41467-019-08631-1
Direct dehydrogenative alkyl Heck-couplings of vinylarenes with umpolung aldehydes catalyzed by nickel
Abstract
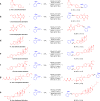
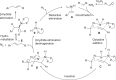
Alkenes are fundamental functionalities in nature and highly useful intermediates in organic synthesis, medicinal chemistry and material sciences. Transition-metal-catalyzed Heck couplings with organic halides as electrophiles have been established as a powerful protocol for the synthesis of this valuable building block. However, the requirement of organic halides and the generation of stoichiometric hazardous halide wastes may cause significant sustainable concerns. The halide-free oxidative Heck alkenylations involving organometallics or arenes as the coupling partners provide a facile and alternative pathway. Nonetheless, stoichiometric amounts of extra oxidant are essential in most cases. Herein, we present a direct dehydrogenative alkyl Heck-coupling reaction under oxidant-free conditions, liberating hydrogen, nitrogen and water as the side products. Excellent regioselectivity is achieved via neighboring oxygen atom coordination. Broad substrate scope, great functional group (ketone, ester, phenol, free amine, amide etc) tolerance and modification of pharmaceutical candidates and biological molecules exemplified its generality and practicability.
Conflict of interest statement
The authors declare no competing interests.
Figures



Comment in
-
[Pd]-Catalyzed para-selective allylation of phenols: access to 4-[(E)-3-aryl/alkylprop-2-enyl]phenols.Org Biomol Chem. 2021 Oct 6;19(38):8259-8263. doi: 10.1039/d1ob01489g. Org Biomol Chem. 2021. PMID: 34532727
Similar articles
-
Photocatalytic Activation of Less Reactive Bonds and Their Functionalization via Hydrogen-Evolution Cross-Couplings.Acc Chem Res. 2018 Oct 16;51(10):2512-2523. doi: 10.1021/acs.accounts.8b00267. Epub 2018 Oct 3. Acc Chem Res. 2018. PMID: 30280898
-
Transition-metal-catalyzed C-N bond forming reactions using organic azides as the nitrogen source: a journey for the mild and versatile C-H amination.Acc Chem Res. 2015 Apr 21;48(4):1040-52. doi: 10.1021/acs.accounts.5b00020. Epub 2015 Mar 30. Acc Chem Res. 2015. PMID: 25821998
-
From α-arylation of olefins to acylation with aldehydes: a journey in regiocontrol of the Heck reaction.Acc Chem Res. 2011 Aug 16;44(8):614-26. doi: 10.1021/ar200053d. Epub 2011 May 25. Acc Chem Res. 2011. PMID: 21612205 Review.
-
Electroreductive Cross-Coupling Reactions: Carboxylation, Deuteration, and Alkylation.Acc Chem Res. 2025 Jan 7;58(1):113-129. doi: 10.1021/acs.accounts.4c00652. Epub 2024 Dec 13. Acc Chem Res. 2025. PMID: 39670841
-
Nickel and cobalt-catalyzed coupling of alkyl halides with alkenes via heck reactions and radical conjugate addition.Mini Rev Med Chem. 2013 May 1;13(6):802-13. doi: 10.2174/1389557511313060003. Mini Rev Med Chem. 2013. PMID: 23544460 Review.
Cited by
-
Visible-light-mediated deoxygenative transformation of 1,2-dicarbonyl compounds through energy transfer process.Nat Commun. 2024 Oct 25;15(1):9240. doi: 10.1038/s41467-024-53635-1. Nat Commun. 2024. PMID: 39455565 Free PMC article.
-
Ruthenium catalyzed β-selective alkylation of vinylpyridines with aldehydes/ketones via N2H4 mediated deoxygenative couplings.Chem Sci. 2020 Dec 30;12(8):2870-2875. doi: 10.1039/d0sc06586b. Chem Sci. 2020. PMID: 34164052 Free PMC article.
-
Visible light mediated photocatalytic [2 + 2] cycloaddition/ring-opening rearomatization cascade of electron-deficient azaarenes and vinylarenes.Commun Chem. 2020 Oct 2;3(1):132. doi: 10.1038/s42004-020-00378-x. Commun Chem. 2020. PMID: 36703325 Free PMC article.
-
Nickel-catalyzed intermolecular oxidative Heck arylation driven by transfer hydrogenation.Nat Commun. 2019 Nov 5;10(1):5025. doi: 10.1038/s41467-019-12949-1. Nat Commun. 2019. PMID: 31690717 Free PMC article.
-
Nickel-Catalyzed Stereoselective Alkenylation of Ketones Mediated by Hydrazine.JACS Au. 2022 Jul 25;2(8):1929-1934. doi: 10.1021/jacsau.2c00320. eCollection 2022 Aug 22. JACS Au. 2022. PMID: 36032538 Free PMC article.
References
-
- Tsutomu M, Kunio M, Atsumu O. Arylation of olefin with aryl iodide catalyzed by palladium. Bull. Chem. Soc. Jpn. 1971;44:581–581.
-
- Heck RF, Nolley JP. Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides. J. Org. Chem. 1972;37:2320–2322.
-
- Beletskaya IP, Cheprakov AV. The Heck reaction as a sharpening stone of palladium catalysis. Chem. Rev. 2000;100:3009–3066. - PubMed
-
- Dounay AB, Overman LE. The asymmetric intramolecular Heck reaction in natural product total synthesis. Chem. Rev. 2003;103:2945–2964. - PubMed
-
- Oestreich M. The Mizoroki-Heck Reaction. Chichester: Wiley & Sons; 2009.
Publication types
LinkOut - more resources
Full Text Sources

