A genetically encoded single-wavelength sensor for imaging cytosolic and cell surface ATP
- PMID: 30755613
- PMCID: PMC6372613
- DOI: 10.1038/s41467-019-08441-5
A genetically encoded single-wavelength sensor for imaging cytosolic and cell surface ATP
Abstract
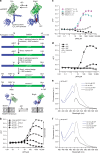
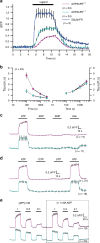
Adenosine 5' triphosphate (ATP) is a universal intracellular energy source and an evolutionarily ancient, ubiquitous extracellular signal in diverse species. Here, we report the generation and characterization of single-wavelength genetically encoded fluorescent sensors (iATPSnFRs) for imaging extracellular and cytosolic ATP from insertion of circularly permuted superfolder GFP into the epsilon subunit of F0F1-ATPase from Bacillus PS3. On the cell surface and within the cytosol, iATPSnFR1.0 responds to relevant ATP concentrations (30 μM to 3 mM) with fast increases in fluorescence. iATPSnFRs can be genetically targeted to specific cell types and sub-cellular compartments, imaged with standard light microscopes, do not respond to other nucleotides and nucleosides, and when fused with a red fluorescent protein function as ratiometric indicators. After careful consideration of their modest pH sensitivity, iATPSnFRs represent promising reagents for imaging ATP in the extracellular space and within cells during a variety of settings, and for further application-specific refinements.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Lohmann K. On the pyrophosphate fraction in muscle. Naturwissenschaften. 1929;17:624–625.
-
- Burnstock G. Purinergic nerves. Pharmacol. Rev. 1972;24:509–581. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

