A method for combining multiple-units readout of optogenetic control with natural stimulation-evoked eyeblink conditioning in freely-moving mice
- PMID: 30755637
- PMCID: PMC6372581
- DOI: 10.1038/s41598-018-37885-w
A method for combining multiple-units readout of optogenetic control with natural stimulation-evoked eyeblink conditioning in freely-moving mice
Abstract
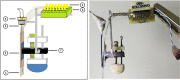
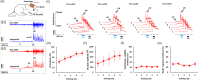
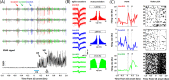
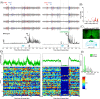
A growing pool of transgenic mice expressing Cre-recombinases, together with Cre-dependent opsin viruses, provide good tools to manipulate specific neural circuits related to eyeblink conditioning (EBC). However, currently available methods do not enable to get fast and precise readout of optogenetic control when the freely-moving mice are receiving EBC training. In the current study, we describe a laser diode (LD)-optical fiber (OF)-Tetrode assembly that allows for simultaneous multiple units recording and optical stimulation. Since the numbers of various cables that require to be connected are minimized, the LD-OF-Tetrode assembly can be combined with CS-US delivery apparatus for revealing the effects of optical stimulation on EBC in freely- moving mice. Moreover, this combination of techniques can be utilized to optogenetically intervene in hippocampal neuronal activities during the post-conditioning sleep in a closed-loop manner. This novel device thus enhances our ability to explore how specific neuronal assembly contributes to associative motor memory in vivo.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Hippocampal Interneurons are Required for Trace Eyeblink Conditioning in Mice.Neurosci Bull. 2021 Aug;37(8):1147-1159. doi: 10.1007/s12264-021-00700-0. Epub 2021 May 15. Neurosci Bull. 2021. PMID: 33991316 Free PMC article.
-
Optetrode: a multichannel readout for optogenetic control in freely moving mice.Nat Neurosci. 2011 Dec 4;15(1):163-70. doi: 10.1038/nn.2992. Nat Neurosci. 2011. PMID: 22138641 Free PMC article.
-
Optogenetic stimulation of mPFC pyramidal neurons as a conditioned stimulus supports associative learning in rats.Sci Rep. 2015 May 14;5:10065. doi: 10.1038/srep10065. Sci Rep. 2015. PMID: 25973929 Free PMC article.
-
[Associative learning: classical eyeblink conditioning with special reference to the role of the higher nervous system].Tanpakushitsu Kakusan Koso. 2004 Feb;49(3 Suppl):493-8. Tanpakushitsu Kakusan Koso. 2004. PMID: 14976778 Review. Japanese. No abstract available.
-
The Anatomy and Physiology of Eyeblink Classical Conditioning.Curr Top Behav Neurosci. 2018;37:297-323. doi: 10.1007/7854_2016_455. Curr Top Behav Neurosci. 2018. PMID: 28025812 Review.
Cited by
-
Hippocampal Interneurons are Required for Trace Eyeblink Conditioning in Mice.Neurosci Bull. 2021 Aug;37(8):1147-1159. doi: 10.1007/s12264-021-00700-0. Epub 2021 May 15. Neurosci Bull. 2021. PMID: 33991316 Free PMC article.
-
Stimulus Generalization in Mice during Pavlovian Eyeblink Conditioning.eNeuro. 2022 Mar 22;9(2):ENEURO.0400-21.2022. doi: 10.1523/ENEURO.0400-21.2022. Print 2022 Mar-Apr. eNeuro. 2022. PMID: 35228312 Free PMC article.
-
Sustained Activity of Hippocampal Parvalbumin-Expressing Interneurons Supports Trace Eyeblink Conditioning in Mice.J Neurosci. 2022 Nov 2;42(44):8343-8360. doi: 10.1523/JNEUROSCI.0834-22.2022. Epub 2022 Sep 27. J Neurosci. 2022. PMID: 36167784 Free PMC article.
-
Enhancing myelin renewal reverses cognitive dysfunction in a murine model of Alzheimer's disease.Neuron. 2021 Jul 21;109(14):2292-2307.e5. doi: 10.1016/j.neuron.2021.05.012. Epub 2021 Jun 7. Neuron. 2021. PMID: 34102111 Free PMC article.
-
Ventromedial Thalamus-Projecting DCN Neurons Modulate Associative Sensorimotor Responses in Mice.Neurosci Bull. 2022 May;38(5):459-473. doi: 10.1007/s12264-021-00810-9. Epub 2022 Jan 6. Neurosci Bull. 2022. PMID: 34989972 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

