Epithelial Cells and Fibroblasts from the Human Female Reproductive Tract Accumulate and Release TFV and TAF to Sustain Inhibition of HIV Infection of CD4+ T cells
- PMID: 30755713
- PMCID: PMC6372694
- DOI: 10.1038/s41598-018-38205-y
Epithelial Cells and Fibroblasts from the Human Female Reproductive Tract Accumulate and Release TFV and TAF to Sustain Inhibition of HIV Infection of CD4+ T cells
Abstract
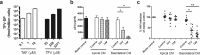
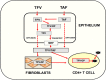
Tenofovir (TFV) treatment of female reproductive tract (FRT) cells results in differential accumulation of intracellular Tenofovir diphosphate (TFV-DP) in different cell types, with greater concentrations in epithelial cells (100-fold) and fibroblasts (10-fold) than in CD4+ T cells. The possibility that TFV-DP accumulation and retention in epithelial cells and fibroblasts may alter TFV availability and protection of CD4+ T cells against HIV infection, prompted us to evaluate TFV and/or Tenofovir alafenamide (TAF) release from FRT cells. Endometrial, endocervical and ectocervical polarized epithelial cells and fibroblasts were pre-loaded with TFV or TAF, and secretions tested for their ability to inhibit HIV infection of activated blood CD4+ T cells. Epithelial cell basolateral secretions (1, 2 and 3 days post-loading), but not apical secretions, suppressed HIV infection of CD4+ T cells, as did secretions from pre-loaded fibroblasts from each site. Intracellular TFV-DP levels in epithelial cells following preloading with TFV or TAF correlated directly with ARV protection of CD4+ T cells from HIV infection. When added apically to epithelial cells, TFV/TAF was released basolaterally, in part through Multidrug Resistant Protein transporters, taken up by fibroblasts and released into secretions to partially protect CD4+ T cells. These findings demonstrate that epithelial cells and fibroblasts release TFV/TAF for use by CD4+ T cells and suggest that the tissue environment plays a major role in the sustained protection against HIV infection.
Conflict of interest statement
The authors declare no competing interests.
Figures

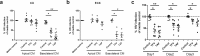


Similar articles
-
Sex hormones regulate tenofovir-diphosphate in female reproductive tract cells in culture.PLoS One. 2014 Jun 30;9(6):e100863. doi: 10.1371/journal.pone.0100863. eCollection 2014. PLoS One. 2014. PMID: 24978212 Free PMC article.
-
Hormonal Contraceptives Differentially Suppress TFV and TAF Inhibition of HIV Infection and TFV-DP in Blood and Genital Tract CD4+ T cells.Sci Rep. 2017 Dec 18;7(1):17697. doi: 10.1038/s41598-017-18078-3. Sci Rep. 2017. PMID: 29255206 Free PMC article.
-
Tenofovir Inhibits Wound Healing of Epithelial Cells and Fibroblasts from the Upper and Lower Human Female Reproductive Tract.Sci Rep. 2017 Apr 3;8:45725. doi: 10.1038/srep45725. Sci Rep. 2017. PMID: 28368028 Free PMC article.
-
Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of Human Immunodeficiency Virus.Antiviral Res. 2016 Jan;125:63-70. doi: 10.1016/j.antiviral.2015.11.009. Epub 2015 Nov 27. Antiviral Res. 2016. PMID: 26640223 Review.
-
Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF).Biochem Pharmacol. 2016 Nov 1;119:1-7. doi: 10.1016/j.bcp.2016.04.015. Epub 2016 Apr 29. Biochem Pharmacol. 2016. PMID: 27133890 Review.
Cited by
-
Comparison of Culturing Methods of Primary Vaginal Fibroblasts.Urogynecology (Phila). 2024 Dec 2:10.1097/SPV.0000000000001612. doi: 10.1097/SPV.0000000000001612. Online ahead of print. Urogynecology (Phila). 2024. PMID: 39621417
-
Localized and Systemic Immune Response in Human Reproductive Tract.Front Cell Infect Microbiol. 2021 Mar 30;11:649893. doi: 10.3389/fcimb.2021.649893. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33859953 Free PMC article. Review.
-
Immune responses in the human female reproductive tract.Immunology. 2020 Jun;160(2):106-115. doi: 10.1111/imm.13136. Epub 2019 Nov 11. Immunology. 2020. PMID: 31630394 Free PMC article. Review.
-
Women for science and science for women: Gaps, challenges and opportunities towards optimizing pre-exposure prophylaxis for HIV-1 prevention.Front Immunol. 2022 Dec 6;13:1055042. doi: 10.3389/fimmu.2022.1055042. eCollection 2022. Front Immunol. 2022. PMID: 36561760 Free PMC article. Review.
-
The Pre-clinical Toolbox of Pharmacokinetics and Pharmacodynamics: in vitro and ex vivo Models.Front Pharmacol. 2019 May 24;10:578. doi: 10.3389/fphar.2019.00578. eCollection 2019. Front Pharmacol. 2019. PMID: 31178736 Free PMC article. Review.
References
-
- WHO. Number of people (all ages) living with HIV: Estimates by WHO region, http://apps.who.int/gho/data/view.main.22100WHO?lang=en (2018).
-
- UNAIDS. Women, girls, gender equality and HIV. (2010).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

