Niraparib activates interferon signaling and potentiates anti-PD-1 antibody efficacy in tumor models
- PMID: 30755715
- PMCID: PMC6372650
- DOI: 10.1038/s41598-019-38534-6
Niraparib activates interferon signaling and potentiates anti-PD-1 antibody efficacy in tumor models
Abstract
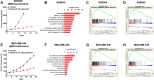
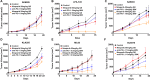
PARP inhibitors have been proven clinically efficacious in platinum-responsive ovarian cancer regardless of BRCA1/2 status and in breast cancers with germline BRCA1/2 mutation. However, resistance to PARP inhibitors may preexist or evolve during treatment in many cancer types and may be overcome by combining PARP inhibitors with other therapies, such as immune checkpoint inhibitors, which confer durable responses and are rapidly becoming the standard of care for multiple tumor types. This study investigated the therapeutic potential of combining niraparib, a highly selective PARP1/2 inhibitor, with anti-PD-1 immune checkpoint inhibitors in preclinical tumor models. Our results indicate that niraparib treatment increases the activity of the type I (alpha) and type II (gamma) interferon pathways and enhances the infiltration of CD8+ cells and CD4+ cells in tumors. When coadministered in immunocompetent models, the combination of niraparib and anti-PD-1 demonstrated synergistic antitumor activities in both BRCA-proficient and BRCA-deficient tumors. Interestingly, mice with tumors cured by niraparib monotherapy completely rejected tumor growth upon rechallenge with the same tumor cell line, suggesting the potential establishment of immune memory in animals treated with niraparib monotherapy. Taken together, our findings uncovered immunomodulatory effects of niraparib that may sensitize tumors to immune checkpoint blockade therapies.
Conflict of interest statement
Z. Wang., K. Sun., Y. Xiao., B. Fen., K. Mikule., J. Hanke., S. Ramaswamy. and J. Wang. are employees and/or shareholders of TESARO, Inc. G. B. Mills receives sponsored research support from TESARO, Inc.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

