Snake venom NAD glycohydrolases: primary structures, genomic location, and gene structure
- PMID: 30755823
- PMCID: PMC6368836
- DOI: 10.7717/peerj.6154
Snake venom NAD glycohydrolases: primary structures, genomic location, and gene structure
Abstract
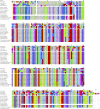
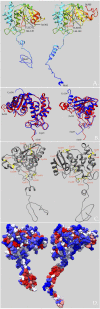
NAD glycohydrolase (EC 3.2.2.5) (NADase) sequences have been identified in 10 elapid and crotalid venom gland transcriptomes, eight of which are complete. These sequences show very high homology, but elapid and crotalid sequences also display consistent differences. As in Aplysia kurodai ADP-ribosyl cyclase and vertebrate CD38 genes, snake venom NADase genes comprise eight exons; however, in the Protobothrops mucrosquamatus genome, the sixth exon is sometimes not transcribed, yielding a shortened NADase mRNA that encodes all six disulfide bonds, but an active site that lacks the catalytic glutamate residue. The function of this shortened protein, if expressed, is unknown. While many vertebrate CD38s are multifunctional, liberating both ADP-ribose and small quantities of cyclic ADP-ribose (cADPR), snake venom CD38 homologs are dedicated NADases. They possess the invariant TLEDTL sequence (residues 144-149) that bounds the active site and the catalytic residue, Glu228. In addition, they possess a disulfide bond (Cys121-Cys202) that specifically prevents ADP-ribosyl cyclase activity in combination with Ile224, in lieu of phenylalanine, which is requisite for ADPR cyclases. In concert with venom phosphodiesterase and 5'-nucleotidase and their ecto-enzyme homologs in prey tissues, snake venom NADases comprise part of an envenomation strategy to liberate purine nucleosides, and particularly adenosine, in the prey, promoting prey immobilization via hypotension and paralysis.
Keywords: 5′-nucleotidase; ADP-ribosyl cyclase; Adenosine; CD38; Exons; NAD glycohydrolase; NADase; Phosphodiesterase; Protobothrops mucrosquamatus genome; Snake venom.
Conflict of interest statement
The authors declare there are no competing interests.
Figures


Similar articles
-
Enzyme properties of Aplysia ADP-ribosyl cyclase: comparison with NAD glycohydrolase of CD38 antigen.J Biochem. 1995 Jan;117(1):125-31. doi: 10.1093/oxfordjournals.jbchem.a124698. J Biochem. 1995. PMID: 7775378
-
Identification of an unusual AT(D)Pase-like activity in multifunctional NAD glycohydrolase from the venom of Agkistrodon acutus.Biochimie. 2009 Feb;91(2):240-51. doi: 10.1016/j.biochi.2008.09.003. Epub 2008 Oct 9. Biochimie. 2009. PMID: 18952139
-
Molecular cloning and functional expression of bovine spleen ecto-NAD+ glycohydrolase: structural identity with human CD38.Biochem J. 2000 Jan 1;345 Pt 1(Pt 1):43-52. doi: 10.1042/bj3450043. Biochem J. 2000. PMID: 10600637 Free PMC article.
-
Ophidian envenomation strategies and the role of purines.Toxicon. 2002 Apr;40(4):335-93. doi: 10.1016/s0041-0101(01)00232-x. Toxicon. 2002. PMID: 11738231 Review.
-
Structure and enzymology of ADP-ribosyl cyclases: conserved enzymes that produce multiple calcium mobilizing metabolites.Curr Mol Med. 2004 May;4(3):249-61. doi: 10.2174/1566524043360708. Curr Mol Med. 2004. PMID: 15101683 Review.
Cited by
-
Solenodon genome reveals convergent evolution of venom in eulipotyphlan mammals.Proc Natl Acad Sci U S A. 2019 Dec 17;116(51):25745-25755. doi: 10.1073/pnas.1906117116. Epub 2019 Nov 26. Proc Natl Acad Sci U S A. 2019. PMID: 31772017 Free PMC article.
References
-
- Aird SD. The role of purine and pyrimidine nucleosides in snake venoms. In: Mackessy SP, editor. Handbook of venoms and toxins of reptiles. CRC Press; Boca Raton: 2009. pp. 393–419.
LinkOut - more resources
Full Text Sources
Research Materials

