Bridging adults and paediatrics with secondary hyperparathyroidism receiving haemodialysis: a pharmacokinetic-pharmacodynamic analysis of cinacalcet
- PMID: 30756425
- PMCID: PMC6533487
- DOI: 10.1111/bcp.13900
Bridging adults and paediatrics with secondary hyperparathyroidism receiving haemodialysis: a pharmacokinetic-pharmacodynamic analysis of cinacalcet
Abstract
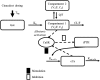
Aims: The aims of this study were to develop a pharmacokinetic (PK) and PK-pharmacodynamic (PK/PD) model of cinacalcet in adults and paediatrics with secondary hyperparathyroidism (SHPT) on dialysis, to test covariates of interest, and to perform simulations to inform dosing in paediatrics with SHPT.
Methods: Cinacalcet PK, intact parathyroid hormone (iPTH) and corrected calcium (cCa) time courses following multiple daily oral doses (1-300 mg) were modelled using a nonlinear mixed effects modelling approach using data from eight clinical studies. Model-based trial simulations, using adult or paediatric titration schemas, predicted efficacy (iPTH change from baseline and proportion achieving iPTH decrease ≥30%) and safety (cCa change from baseline and proportion achieving cCa ≤8.4 mg/dL) endpoints at 24 weeks.
Results: Cinacalcet PK parameters were described by a two-compartment linear model with delayed first-order absorption-elimination (apparent clearance = 287.74 L h-1 ). Simulations suggested that paediatric starting doses (1, 2.5, 5, 10 and 15 mg) would provide PK exposures less than or similar to a 30 mg adult dose. The titrated dose simulations suggested that the mean (prediction interval) proportion of paediatric and adult subjects achieving ≥30% reduction in iPTH from baseline at Week 24 was 49% (36%, 62%), and 70.1% (62.5%, 77%), respectively. Additionally, the mean (confidence interval) proportion of paediatric and adult subjects achieving cCa ≤8.4 mg dL-1 at Week 24 was 8% (2%, 18%) and 23.6% (17.5%, 30.5%), respectively.
Conclusions: Model-based simulations showed that the paediatric cinacalcet starting dose (0.2 mg kg-1 ), titrated to effect, would provide the desired PD efficacy (PTH suppression <30%) while minimizing safety concerns (hypocalcaemia).
Keywords: PK/PD; chronic kidney disease; dialysis; modelling and simulation; paediatrics.
© 2019 Amgen Inc. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
P.C., W.S. and A.N. are employees of Amgen Inc. M.M., currently with Vertex Pharmaceuticals, was an employee of Amgen at the time the work was completed. P.O.G. was a consultant on this analysis.
Figures



References
-
- Ketteler M, Block GA, Evenepoel P, et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease‐Mineral and Bone Disorder (CKD‐MBD) Guideline Update: What's changed and why it matters. Kidney Int. 2017;92(1):26‐36. - PubMed
-
- Goodman WG. The consequences of uncontrolled secondary hyperparathyroidism and its treatment in chronic kidney disease. Semin Dial. 2004;17(3):209‐216. - PubMed
-
- Silver J, Naveh‐Many T. Regulation of parathyroid hormone synthesis and secretion. Semin Nephrol. 1994;14(2):175‐194. - PubMed
-
- Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: Pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6(4):913‐921. - PubMed
-
- Goodman WG, Goldin J, Kuizon BD, et al. Coronary‐artery calcification in young adults with end‐stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342(20):1478‐1483. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

