Effects of Nrf2 deficiency on mitochondrial oxidative stress in aged skeletal muscle
- PMID: 30756520
- PMCID: PMC6372533
- DOI: 10.14814/phy2.13998
Effects of Nrf2 deficiency on mitochondrial oxidative stress in aged skeletal muscle
Abstract
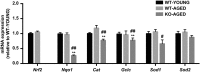
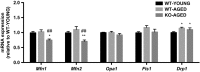
Oxidative stress and mitochondrial dysfunction are associated with the aging process. However, the role of nuclear factor erythroid 2 -related factor 2 (Nrf2) in skeletal muscle during aging remains to be clarified. In the current study, we assessed whether the lack of Nrf2, which is known as a master regulator of redox homeostasis, promotes age-related mitochondrial dysfunction and muscle atrophy in skeletal muscle. Here, we demonstrated that mitochondrial 4-hydroxynonenal and protein carbonyls, markers of oxidative stress, were robustly elevated in aged Nrf2 knockout (KO) mice because of the decreased expression of Nrf2-target antioxidant genes. Mitochondrial respiration declined with aging; however, there was no difference between Nrf2 KO and age-matched WT mice. Similarly, cytochrome c oxidase activity was lower in aged WT and Nrf2 KO mice compared with young WT mice. The expression of Mfn1 and Mfn2 mRNA was lower in aged Nrf2 KO muscle. Mitochondrial reactive oxygen species production per oxygen consumed was elevated in aged Nrf2 KO mice. There was no effect of Nrf2 KO on muscle mass normalized to body weight. These results suggest that Nrf2 deficiency exacerbates age-related mitochondrial oxidative stress but does not affect the decline of respiratory function in skeletal muscle.
Keywords: Aging; mitochondria; oxidative stress; skeletal muscle.
© 2019 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures
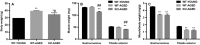
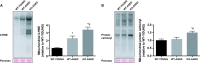


Similar articles
-
Nrf2 deficiency exacerbates frailty and sarcopenia by impairing skeletal muscle mitochondrial biogenesis and dynamics in an age-dependent manner.Exp Gerontol. 2019 May;119:61-73. doi: 10.1016/j.exger.2019.01.022. Epub 2019 Jan 25. Exp Gerontol. 2019. PMID: 30690066
-
Muscle-specific PGC-1α modulates mitochondrial oxidative stress in aged sarcopenia through regulating Nrf2.Exp Gerontol. 2024 Aug;193:112468. doi: 10.1016/j.exger.2024.112468. Epub 2024 May 28. Exp Gerontol. 2024. PMID: 38801840
-
Nrf2 deficiency does not affect denervation-induced alterations in mitochondrial fission and fusion proteins in skeletal muscle.Physiol Rep. 2016 Dec;4(24):e13064. doi: 10.14814/phy2.13064. Physiol Rep. 2016. PMID: 28039408 Free PMC article.
-
Aging-associated Aberrant Mitochondrial Redox Signaling, Physical Activity, and Sarcopenia.Curr Aging Sci. 2025;18(2):120-131. doi: 10.2174/0118746098315667240606052523. Curr Aging Sci. 2025. PMID: 38920079 Review.
-
Mitochondria, muscle health, and exercise with advancing age.Physiology (Bethesda). 2015 May;30(3):208-23. doi: 10.1152/physiol.00039.2014. Physiology (Bethesda). 2015. PMID: 25933821 Review.
Cited by
-
Accumulation of polyunsaturated fatty acid-derived metabolites in the sarcopenic muscle of aging mice.Geriatr Gerontol Int. 2023 Apr;23(4):297-303. doi: 10.1111/ggi.14561. Epub 2023 Feb 22. Geriatr Gerontol Int. 2023. PMID: 36811314 Free PMC article.
-
Increased Circulating Levels of Interleukin-6 Affect the Redox Balance in Skeletal Muscle.Oxid Med Cell Longev. 2019 Nov 16;2019:3018584. doi: 10.1155/2019/3018584. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31827671 Free PMC article.
-
Regulation of KCNMA1 transcription by Nrf2 in coronary arterial smooth muscle cells.J Mol Cell Cardiol. 2020 Mar;140:68-76. doi: 10.1016/j.yjmcc.2020.03.001. Epub 2020 Mar 5. J Mol Cell Cardiol. 2020. PMID: 32147517 Free PMC article.
-
Nrf2 deficiency promotes the increasing trend of autophagy during aging in skeletal muscle: a potential mechanism for the development of sarcopenia.Aging (Albany NY). 2020 Apr 3;12(7):5977-5991. doi: 10.18632/aging.102990. Epub 2020 Apr 3. Aging (Albany NY). 2020. PMID: 32244226 Free PMC article.
-
Redox Signaling and Sarcopenia: Searching for the Primary Suspect.Int J Mol Sci. 2021 Aug 22;22(16):9045. doi: 10.3390/ijms22169045. Int J Mol Sci. 2021. PMID: 34445751 Free PMC article. Review.
References
-
- Carter, H. N. , Chen C. C., and Hood D. A.. 2015. Mitochondria, muscle health, and exercise with advancing age. Physiology 30:208–223. - PubMed
-
- Chabi, B. , Ljubicic V., Menzies K. J., Huang J. H., Saleem A., and Hood D. A.. 2008. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell 7:2–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

