Characterization of membrane vesicles released by Mycobacterium avium in response to environment mimicking the macrophage phagosome
- PMID: 30757918
- PMCID: PMC6479280
- DOI: 10.2217/fmb-2018-0249
Characterization of membrane vesicles released by Mycobacterium avium in response to environment mimicking the macrophage phagosome
Abstract
Aim: To investigate the formation of Mycobacterium avium membrane vesicles (MVs) within macrophage phagosomes.
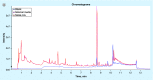
Materials & methods: A phagosome model was utilized to characterize proteomics and lipidomics of MVs. A click chemistry-based enrichment assay was employed to examine the presence of MV proteins in the cytosol of host cells.
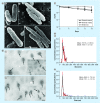
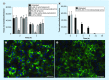
Results: Exposure to metals at concentrations present in phagosomes triggers formation of bacterial MVs. Proteomics identified several virulence factors, including enzymes involved in the cell wall synthesis, lipid and fatty acid metabolism. Some of MV proteins were also identified in the cytosol of infected macrophages. MVs harbor dsDNA.
Conclusion: M. avium produces MVs within phagosomes. MVs carry products with potential roles in modulation of host immune defenses and intracellular survival.
Keywords: AHA; bioorthogonal metabolic labeling; macrophages; membrane vesicles; minimal medium; phagosome model.
Conflict of interest statement
This work was supported by the Oregon State University Foundation FS062E-VF01 (L Danelishvili) and by the Oregon State University Incentive Programs VBS330-001100 (L Danelishvili). The proteomic sequencing was conducted by the Oregon State University (OSU) Mass Spectrometry Center supported in part by OSU's Research Office and institutional funds. The procurement of the Orbitrap Fusion Lumos was made possible by National Institutes of Health grant S10 OD020111. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures




Similar articles
-
Identification of Mycobacterium avium subsp. hominissuis secreted proteins using an in vitro system mimicking the phagosomal environment.BMC Microbiol. 2016 Nov 9;16(1):270. doi: 10.1186/s12866-016-0889-y. BMC Microbiol. 2016. PMID: 27829372 Free PMC article.
-
Iron transport into mycobacterium avium-containing phagosomes from an Nramp1(Gly169)-transfected RAW264.7 macrophage cell line.J Leukoc Biol. 2001 Jan;69(1):43-9. J Leukoc Biol. 2001. PMID: 11200066
-
The interaction between Mycobacterium and the macrophage analyzed by two-dimensional polyacrylamide gel electrophoresis.Electrophoresis. 1997 Dec;18(14):2558-65. doi: 10.1002/elps.1150181411. Electrophoresis. 1997. PMID: 9527485
-
Role of host- and pathogen-associated lipids in directing the immune response in mycobacterial infections, with emphasis on Mycobacterium avium subsp. paratuberculosis.Crit Rev Microbiol. 2016;42(2):262-75. doi: 10.3109/1040841X.2014.932327. Epub 2014 Aug 28. Crit Rev Microbiol. 2016. PMID: 25163812 Review.
-
The many niches and strategies used by pathogenic mycobacteria for survival within host macrophages.Immunobiology. 2009;214(7):526-42. doi: 10.1016/j.imbio.2008.12.005. Epub 2009 Mar 3. Immunobiology. 2009. PMID: 19261352 Review.
Cited by
-
Orchestration of human macrophage NLRP3 inflammasome activation by Staphylococcus aureus extracellular vesicles.Proc Natl Acad Sci U S A. 2020 Feb 11;117(6):3174-3184. doi: 10.1073/pnas.1915829117. Epub 2020 Jan 27. Proc Natl Acad Sci U S A. 2020. PMID: 31988111 Free PMC article.
-
Surface-Shaving Proteomics of Mycobacterium marinum Identifies Biofilm Subtype-Specific Changes Affecting Virulence, Tolerance, and Persistence.mSystems. 2021 Jun 29;6(3):e0050021. doi: 10.1128/mSystems.00500-21. Epub 2021 Jun 22. mSystems. 2021. PMID: 34156290 Free PMC article.
-
The role of antibiotic-derived mycobacterial vesicles in tuberculosis pathogenesis.Sci Rep. 2024 Nov 15;14(1):28198. doi: 10.1038/s41598-024-79215-3. Sci Rep. 2024. PMID: 39548211 Free PMC article.
-
Meeting the challenges of NTM-PD from the perspective of the organism and the disease process: innovations in drug development and delivery.Respir Res. 2022 Dec 24;23(1):376. doi: 10.1186/s12931-022-02299-w. Respir Res. 2022. PMID: 36566170 Free PMC article. Review.
-
Genetic Involvement of Mycobacterium avium Complex in the Regulation and Manipulation of Innate Immune Functions of Host Cells.Int J Mol Sci. 2021 Mar 16;22(6):3011. doi: 10.3390/ijms22063011. Int J Mol Sci. 2021. PMID: 33809463 Free PMC article. Review.
References
-
- Armstrong D, Gold JW, Dryjanski J, et al. Treatment of infections in patients with the acquired immunodeficiency syndrome. Ann. Intern. Med. 1985;103(5):738–743. - PubMed
-
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007;175(4):367–416. - PubMed
-
- Field SK, Fisher D, Cowie RL. Mycobacterium avium complex pulmonary disease in patients without HIV infection. Chest. 2004;126(2):566–581. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
