Neurobiology of maternal regulation of infant fear: the role of mesolimbic dopamine and its disruption by maltreatment
- PMID: 30758321
- PMCID: PMC6784970
- DOI: 10.1038/s41386-019-0340-9
Neurobiology of maternal regulation of infant fear: the role of mesolimbic dopamine and its disruption by maltreatment
Abstract
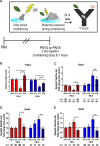
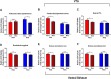
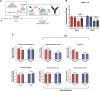
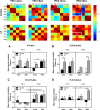
Child development research highlights caregiver regulation of infant physiology and behavior as a key feature of early life attachment, although mechanisms for maternal control of infant neural circuits remain elusive. Here we explored the neurobiology of maternal regulation of infant fear using neural network and molecular levels of analysis in a rodent model. Previous research has shown maternal suppression of amygdala-dependent fear learning during a sensitive period. Here we characterize changes in neural networks engaged during maternal regulation and the transition to infant self-regulation. Metabolic mapping of 2-deoxyglucose uptake during odor-shock conditioning in postnatal day (PN)14 rat pups showed that maternal presence blocked fear learning, disengaged mesolimbic circuitry, basolateral amygdala (BLA), and plasticity-related AMPA receptor subunit trafficking. At PN18, when maternal presence only socially buffers threat learning (similar to social modulation in adults), maternal presence failed to disengage the mesolimbic dopaminergic system, and failed to disengage both the BLA and plasticity-related AMPA receptor subunit trafficking. Further, maternal presence failed to block threat learning at PN14 pups following abuse, and mesolimbic dopamine engagement and AMPA were not significantly altered by maternal presence-analogous to compromised maternal regulation of children in abusive relationships. Our results highlight three key features of maternal regulation: (1) maternal presence blocks fear learning and amygdala plasticity through age-dependent suppression of amygdala AMPA receptor subunit trafficking, (2) maternal presence suppresses engagement of brain regions within the mesolimbic dopamine circuit, and (3) early-life abuse compromises network and molecular biomarkers of maternal regulation, suggesting reduced social scaffolding of the brain.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Dual circuitry for odor-shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala.J Neurosci. 2006 Jun 21;26(25):6737-48. doi: 10.1523/JNEUROSCI.0499-06.2006. J Neurosci. 2006. PMID: 16793881 Free PMC article.
-
Developing a neurobehavioral animal model of infant attachment to an abusive caregiver.Biol Psychiatry. 2010 Jun 15;67(12):1137-45. doi: 10.1016/j.biopsych.2009.12.019. Epub 2010 Feb 16. Biol Psychiatry. 2010. PMID: 20163787 Free PMC article.
-
Conditioned fear is modulated by D2 receptor pathway connecting the ventral tegmental area and basolateral amygdala.Neurobiol Learn Mem. 2011 Jan;95(1):37-45. doi: 10.1016/j.nlm.2010.10.005. Epub 2010 Oct 16. Neurobiol Learn Mem. 2011. PMID: 20955808
-
The neurobiology of safety and threat learning in infancy.Neurobiol Learn Mem. 2017 Sep;143:49-58. doi: 10.1016/j.nlm.2016.10.015. Epub 2016 Nov 4. Neurobiol Learn Mem. 2017. PMID: 27826033 Free PMC article. Review.
-
The development and neurobiology of infant attachment and fear.Dev Neurosci. 2012;34(2-3):101-14. doi: 10.1159/000336732. Epub 2012 May 8. Dev Neurosci. 2012. PMID: 22571921 Free PMC article. Review.
Cited by
-
Developmental Shifts in Amygdala Function.Curr Top Behav Neurosci. 2024 Nov 16:10.1007/7854_2024_538. doi: 10.1007/7854_2024_538. Online ahead of print. Curr Top Behav Neurosci. 2024. PMID: 39546164
-
Adverse caregiving in infancy blunts neural processing of the mother.Nat Commun. 2020 Feb 28;11(1):1119. doi: 10.1038/s41467-020-14801-3. Nat Commun. 2020. PMID: 32111822 Free PMC article.
-
Amygdala circuit transitions supporting developmentally-appropriate social behavior.Neurobiol Learn Mem. 2023 May;201:107762. doi: 10.1016/j.nlm.2023.107762. Epub 2023 Apr 26. Neurobiol Learn Mem. 2023. PMID: 37116857 Free PMC article. Review.
-
Early Life Trauma Has Lifelong Consequences for Sleep And Behavior.Sci Rep. 2019 Nov 13;9(1):16701. doi: 10.1038/s41598-019-53241-y. Sci Rep. 2019. PMID: 31723235 Free PMC article.
-
Elevated infant cortisol is necessary but not sufficient for transmission of environmental risk to infant social development: Cross-species evidence of mother-infant physiological social transmission.Dev Psychopathol. 2020 Dec;32(5):1696-1714. doi: 10.1017/S0954579420001455. Dev Psychopathol. 2020. PMID: 33427190 Free PMC article.
References
-
- Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: diversity, mechanisms, and functions. Front Neuroendocrinol. 2009;30:470–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32-MH112232/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)/International
- R25 GM060665/GM/NIGMS NIH HHS/United States
- T32-MH019524/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)/International
- R37 HD083217/HD/NICHD NIH HHS/United States
- MH109779/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)/International
LinkOut - more resources
Full Text Sources

