Effects of chloride content of intravenous crystalloid solutions in critically ill adult patients: a meta-analysis with trial sequential analysis of randomized trials
- PMID: 30758680
- PMCID: PMC6374495
- DOI: 10.1186/s13613-019-0506-y
Effects of chloride content of intravenous crystalloid solutions in critically ill adult patients: a meta-analysis with trial sequential analysis of randomized trials
Abstract
Background: Intravenous crystalloid solutions are administered commonly for critically ill patients. We performed this meta-analysis of randomized trials with trial sequential analysis (TSA) to evaluate effects of chloride content of intravenous crystalloid solutions on clinical outcomes among critically ill adult patients.
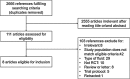
Methods: Electronic databases were searched up to June 1, 2018, for randomized trials of use of balanced crystalloids versus 0.9% saline solutions in critically ill adult patients. The outcome variables included mortality, renal outcomes, serum content alterations and organ function. Subgroup analysis was conducted according to patient settings, types or volume of crystalloid fluid, or among sepsis versus non-sepsis, TBI versus non-TBI or subpopulations by the categories of baseline kidney function. Random errors were evaluated by trial sequential analysis.
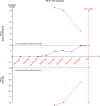
Results: Eight studies with 19,301 patients were analyzed. A trend of in-hospital survival benefit with no statistical difference could be observed with balanced crystalloids compared with 0.9% saline (RR 0.92, 95% CI 0.85-1.0, p = 0.06). The use of balanced crystalloid solutions was associated with longer RRT-free days (SMD 0.09, 95% CI 0.06-0.12, p < 0.001), less risk of increase in serum concentrations of chloride (SMD - 1.23, 95% CI - 1.59 to - 0.87, p < 0.001) and sodium (SMD - 1.28, 95% CI - 1.65 to - 0.92, p < 0.001), less risk of decline in serum base deficit (SMD - 0.58, 95% CI - 0.98 to - 0.18, p = 0.004), longer ventilator-free days (SMD 0.08, 95% CI 0.05-0.11, p < 0.001) and vasopressor-free days (SMD 0.04, 95% CI 0.00-0.07, p = 0.02). Subgroup analysis showed that balanced crystalloid solutions were associated with a reduced in-hospital mortality rate among septic patients (RR 0.86, 95% CI 0.75-0.98; p = 0.02) and non-traumatic brain injury patients (RR 0.90, 95% CI 0.82-0.99, p = 0.02), while the TSA results indicated a larger sample size is still in need.
Conclusions: Limited evidence supported statistical survival benefit with balanced crystalloid solutions, while it benefited in reducing organ support duration and fluctuations in serum electrolyte and base excess and was associated with decreased in-hospital mortality in subpopulation with sepsis and non-TBI. Large-scale rigorous randomized trials with better designs are needed to provide robust evidence for clinical management. Trial registration The protocol for this meta-analysis was registered on PROSPERO: International prospective register of systematic reviews (CRD42018102661), https://www.crd.york.ac.uk/prospero/#recordDetails.
Keywords: 0.9% Saline; Critically ill patients; In-hospital mortality; Lactated Ringers; Plasma-Lyte 148; Renal outcome.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

