Primary immune thrombocytopenia (ITP) treated with romiplostim in routine clinical practice: retrospective study from the United Kingdom ITP Registry
- PMID: 30758874
- PMCID: PMC6850028
- DOI: 10.1111/ejh.13221
Primary immune thrombocytopenia (ITP) treated with romiplostim in routine clinical practice: retrospective study from the United Kingdom ITP Registry
Abstract
Background: Romiplostim is a thrombopoietin-mimetic peptibody for adult refractory chronic immune thrombocytopenia (ITP). We aimed to describe ITP patients receiving romiplostim, platelet counts and romiplostim usage in UK clinical practice.
Methods: This was a retrospective cohort study of patients in the UKITP Registry who received romiplostim between October 2009 and January 2015, including data up to 6 months before romiplostim initiation through follow-up.
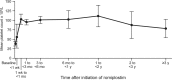
Results: Of 1440 patients in the UKITP Registry, 118 adults with primary ITP were eligible. Before romiplostim, 22% had splenectomy, 12% received platelet transfusion, 97% received ≥ 1 different ITP medication and 77% received ≥ 3. Most patients (73%) initiated romiplostim ≥ 1 year after ITP diagnosis (chronic phase). The mean duration of romiplostim treatment was 5.7 (SE 0.9) months, and the median was 1.4 months (IQR: 0.2, 6.5). Mean platelet count before romiplostim was 38 × 109 /L, rising to 103 × 109 /L within 1 month, and remaining 50-150 × 109 /L through up to 3 years of follow-up. After romiplostim, 4% of patients had splenectomy, 6% received platelet transfusion, and 57% received just one ITP medication other than romiplostim.
Conclusion: The study provides valuable insights into the real-world use of romiplostim in primary ITP in routine practice and highlighted the timing of romiplostim initiation at different ITP disease phases.
Keywords: bleeding; idiopathic thrombocytopenic purpura; platelet; primary immune thrombocytopenia; romiplostim; thrombopoietin receptor agonists.
© 2019 The Authors. European Journal of Haematology Published by John Wiley & Sons Ltd.
Figures
References
-
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386‐2393. - PubMed
-
- Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168‐186. - PubMed
-
- Cohen YC, Djulbegovic B, Shamai‐Lubovitz O, Mozes B. The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistent low platelet counts. Arch Intern Med. 2000;160(11):1630‐1638. - PubMed
-
- Neylon AJ, Saunders PW, Howard MR, Proctor SJ, Taylor PR, Northern Region Haematology G . Clinically significant newly presenting autoimmune thrombocytopenic purpura in adults: a prospective study of a population‐based cohort of 245 patients. Br J Haematol 2003;122(6):966‐974. - PubMed
-
- Moulis G, Palmaro A, Montastruc JL, Godeau B, Lapeyre‐Mestre M, Sailler L. Epidemiology of incident immune thrombocytopenia: a nationwide population‐based study in France. Blood. 2014;124(22):3308‐3315. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources