Extracellular vesicles: another compartment for the second messenger, cyclic adenosine monophosphate
- PMID: 30758991
- PMCID: PMC6483015
- DOI: 10.1152/ajplung.00282.2018
Extracellular vesicles: another compartment for the second messenger, cyclic adenosine monophosphate
Abstract
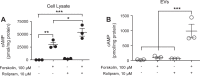
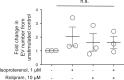
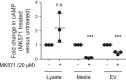
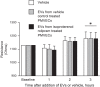
The second messenger, cAMP, is highly compartmentalized to facilitate signaling specificity. Extracellular vesicles (EVs) are submicron, intact vesicles released from many cell types that can act as biomarkers or be involved in cell-to-cell communication. Although it is well recognized that EVs encapsulate functional proteins and RNAs/miRNAs, currently it is unclear whether cyclic nucleotides are encapsulated within EVs to provide an additional second messenger compartment. Using ultracentrifugation, EVs were isolated from the culture medium of unstimulated systemic and pulmonary endothelial cells. EVs were also isolated from pulmonary microvascular endothelial cells (PMVECs) following stimulation of transmembrane adenylyl cyclase (AC) in the presence or absence of the phosphodiesterase 4 inhibitor rolipram over time. Whereas cAMP was detected in EVs isolated from endothelial cells derived from different vascular beds, it was highest in EVs isolated from PMVECs. Treatment of PMVECs with agents that increase near-membrane cAMP led to an increase in cAMP within corresponding EVs, yet there was no increase in EV number. Elevated cell cAMP, measured by whole cell measurements, peaked 15 min after treatment, yet in EVs the peak increase in cAMP was delayed until 60 min after cell stimulation. Cyclic AMP was also increased in EVs collected from the perfusate of isolated rat lungs stimulated with isoproterenol and rolipram, thus corroborating cell culture findings. When added to unperturbed confluent PMVECs, EVs containing elevated cAMP were not barrier disruptive like cytosolic cAMP but maintained monolayer resistance. In conclusion, PMVECs release EVs containing cAMP, providing an additional compartment to cAMP signaling.
Keywords: cyclic adenosine monophosphate; endothelium; extracellular vesicles; isolated lung; phosphodiesterase.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





References
-
- Bakouboula B, Morel O, Faure A, Zobairi F, Jesel L, Trinh A, Zupan M, Canuet M, Grunebaum L, Brunette A, Desprez D, Chabot F, Weitzenblum E, Freyssinet JM, Chaouat A, Toti F. Procoagulant membrane microparticles correlate with the severity of pulmonary arterial hypertension. Am J Respir Crit Care Med 177: 536–543, 2008. doi:10.1164/rccm.200706-840OC. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

