The primate-specific peptide Y-P30 regulates morphological maturation of neocortical dendritic spines
- PMID: 30759095
- PMCID: PMC6373909
- DOI: 10.1371/journal.pone.0211151
The primate-specific peptide Y-P30 regulates morphological maturation of neocortical dendritic spines
Abstract
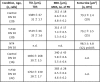
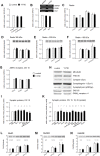
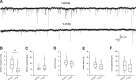
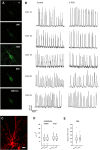
The 30-amino acid peptide Y-P30 corresponds to the N-terminus of the primate-specific, sweat gland-derived dermcidin prepropeptide. Previous work has revealed that Y-P30 enhances the interaction of pleiotrophin and syndecans-2/3, and thus represents a natural ligand to study this signaling pathway. In immature neurons, Y-P30 activates the c-Src and p42/44 ERK kinase pathway, increases the amount of F-actin in axonal growth cones, and promotes neuronal survival, cell migration and axonal elongation. The action of Y-P30 on axonal growth requires syndecan-3 and heparan sulfate side chains. Whether Y-P30 has the potential to influence dendrites and dendritic protrusions has not been explored. The latter is suggested by the observations that syndecan-2 expression increases during postnatal development, that syndecan-2 becomes enriched in dendritic spines, and that overexpression of syndecan-2 in immature neurons results in a premature morphological maturation of dendritic spines. Here, analysing rat cortical pyramidal and non-pyramidal neurons in organotypic cultures, we show that Y-P30 does not alter the development of the dendritic arborization patterns. However, Y-P30 treatment decreases the density of apical, but not basal dendritic protrusions at the expense of the filopodia. Analysis of spine morphology revealed an unchanged mushroom/stubby-to-thin spine ratio and a shortening of the longest decile of dendritic protrusions. Whole-cell recordings from cortical principal neurons in dissociated cultures grown in the presence of Y-P30 demonstrated a decrease in the frequency of glutamatergic mEPSCs. Despite these differences in protrusion morphology and synaptic transmission, the latter likely attributable to presynaptic effects, calcium event rate and amplitude recorded in pyramidal neurons in organotypic cultures were not altered by Y-P30 treatment. Together, our data suggest that Y-P30 has the capacity to decelerate spinogenesis and to promote morphological, but not synaptic, maturation of dendritic protrusions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Y-P30 promotes axonal growth by stabilizing growth cones.Brain Struct Funct. 2015 Jul;220(4):1935-50. doi: 10.1007/s00429-014-0764-2. Epub 2014 Apr 13. Brain Struct Funct. 2015. PMID: 24728870
-
Calcium influx and postsynaptic proteins coordinate the dendritic filopodium-spine transition.Dev Neurobiol. 2014 Oct;74(10):1011-29. doi: 10.1002/dneu.22181. Epub 2014 Apr 28. Dev Neurobiol. 2014. PMID: 24753440
-
Type I TARPs promote dendritic growth of early postnatal neocortical pyramidal cells in organotypic cultures.Development. 2014 Apr;141(8):1737-48. doi: 10.1242/dev.099697. Epub 2014 Mar 25. Development. 2014. PMID: 24667327
-
Emerging Roles of Filopodia and Dendritic Spines in Motoneuron Plasticity during Development and Disease.Neural Plast. 2016;2016:3423267. doi: 10.1155/2016/3423267. Epub 2015 Dec 30. Neural Plast. 2016. PMID: 26843990 Free PMC article. Review.
-
Structural modulation of dendritic spines during synaptic plasticity.Neuroscientist. 2012 Aug;18(4):326-41. doi: 10.1177/1073858411407206. Epub 2011 Jun 13. Neuroscientist. 2012. PMID: 21670426 Review.
Cited by
-
Pleiotrophin: Activity and mechanism.Adv Clin Chem. 2020;98:51-89. doi: 10.1016/bs.acc.2020.02.003. Epub 2020 Mar 12. Adv Clin Chem. 2020. PMID: 32564788 Free PMC article. Review.
-
A single-cell transcriptomic atlas of human lens epithelium: identification and functional insights into lens stem/progenitor cells.Stem Cell Res Ther. 2025 Jul 1;16(1):333. doi: 10.1186/s13287-025-04436-w. Stem Cell Res Ther. 2025. PMID: 40598599 Free PMC article.
-
GluN2B but Not GluN2A for Basal Dendritic Growth of Cortical Pyramidal Neurons.Front Neuroanat. 2020 Nov 13;14:571351. doi: 10.3389/fnana.2020.571351. eCollection 2020. Front Neuroanat. 2020. PMID: 33281565 Free PMC article.
-
Distinct regulation of bioenergetics and translation by group I mGluR and NMDAR.EMBO Rep. 2020 Jun 4;21(6):e48037. doi: 10.15252/embr.201948037. Epub 2020 Apr 29. EMBO Rep. 2020. PMID: 32351028 Free PMC article.
References
-
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28(6):517–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

