Molecular and physiological control of adventitious rooting in cuttings: phytohormone action meets resource allocation
- PMID: 30759178
- PMCID: PMC6589513
- DOI: 10.1093/aob/mcy234
Molecular and physiological control of adventitious rooting in cuttings: phytohormone action meets resource allocation
Abstract
Background: Adventitious root (AR) formation in excised plant parts is a bottleneck for survival of isolated plant fragments. AR formation plays an important ecological role and is a critical process in cuttings for the clonal propagation of horticultural and forestry crops. Therefore, understanding the regulation of excision-induced AR formation is essential for sustainable and efficient utilization of plant genetic resources.
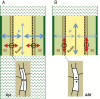
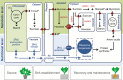
Scope: Recent studies of plant transcriptomes, proteomes and metabolomes, and the use of mutants and transgenic lines have significantly expanded our knowledge concerning excision-induced AR formation. Here, we integrate new findings regarding AR formation in the cuttings of diverse plant species. These findings support a new system-oriented concept that the phytohormone-controlled reprogramming and differentiation of particular responsive cells in the cutting base interacts with a co-ordinated reallocation of plant resources within the whole cutting to initiate and drive excision-induced AR formation. Master control by auxin involves diverse transcription factors and mechanically sensitive microtubules, and is further linked to ethylene, jasmonates, cytokinins and strigolactones. Hormone functions seem to involve epigenetic factors and cross-talk with metabolic signals, reflecting the nutrient status of the cutting. By affecting distinct physiological units in the cutting, environmental factors such as light, nitrogen and iron modify the implementation of the genetically controlled root developmental programme.
Conclusion: Despite advanced research in the last decade, important questions remain open for future investigations on excision-induced AR formation. These concern the distinct roles and interactions of certain molecular, hormonal and metabolic factors, as well as the functional equilibrium of the whole cutting in a complex environment. Starting from model plants, cell type- and phase-specific monitoring of controlling processes and modification of gene expression are promising methodologies that, however, need to be integrated into a coherent model of the whole system, before research findings can be translated to other crops.
Keywords: Adventitious rooting; auxin; carbohydrates; chromatin; genetic; mechanical stress; mineral; plant hormones; root; signalling; source–sink; wound response.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Annals of Botany Company.
Figures


Similar articles
-
Auxin regulates adventitious root formation in tomato cuttings.BMC Plant Biol. 2019 Oct 21;19(1):435. doi: 10.1186/s12870-019-2002-9. BMC Plant Biol. 2019. PMID: 31638898 Free PMC article.
-
Adventitious rooting declines with the vegetative to reproductive switch and involves a changed auxin homeostasis.J Exp Bot. 2015 Mar;66(5):1437-52. doi: 10.1093/jxb/eru499. Epub 2014 Dec 24. J Exp Bot. 2015. PMID: 25540438 Free PMC article.
-
Plant Hormone Homeostasis, Signaling, and Function during Adventitious Root Formation in Cuttings.Front Plant Sci. 2016 Mar 31;7:381. doi: 10.3389/fpls.2016.00381. eCollection 2016. Front Plant Sci. 2016. PMID: 27064322 Free PMC article. Review.
-
Integrated transcriptome and hormonal analysis of naphthalene acetic acid-induced adventitious root formation of tea cuttings (Camellia sinensis).BMC Plant Biol. 2022 Jul 4;22(1):319. doi: 10.1186/s12870-022-03701-x. BMC Plant Biol. 2022. PMID: 35787241 Free PMC article.
-
Adventitious Root Formation in Cuttings: Insights from Arabidopsis and Prospects for Woody Plants.Biomolecules. 2025 Jul 28;15(8):1089. doi: 10.3390/biom15081089. Biomolecules. 2025. PMID: 40867534 Free PMC article. Review.
Cited by
-
Anatomy of recalcitrance: integrated imaging and spectroscopy reveal features of hard-to-root rose cuttings.J Exp Bot. 2024 Aug 28;75(16):4680-4683. doi: 10.1093/jxb/erae218. J Exp Bot. 2024. PMID: 39192695 Free PMC article.
-
Transcriptome Analysis Reveals Multiple Genes and Complex Hormonal-Mediated Interactions with PEG during Adventitious Root Formation in Apple.Int J Mol Sci. 2022 Jan 17;23(2):976. doi: 10.3390/ijms23020976. Int J Mol Sci. 2022. PMID: 35055162 Free PMC article.
-
Role of auxin homeostasis and response in nitrogen limitation and dark stimulation of adventitious root formation in petunia cuttings.Ann Bot. 2019 Nov 27;124(6):1053-1066. doi: 10.1093/aob/mcz095. Ann Bot. 2019. PMID: 31181150 Free PMC article.
-
Integration of Phenotype and Hormone Data during Adventitious Rooting in Carnation (Dianthus caryophyllus L.) Stem Cuttings.Plants (Basel). 2019 Jul 15;8(7):226. doi: 10.3390/plants8070226. Plants (Basel). 2019. PMID: 31311180 Free PMC article.
-
Unraveling the genetic basis of Rhizobium rhizogenes-mediated transformation and hairy root formation in rose using a genome-wide association study.Plant Cell Rep. 2024 Dec 3;43(12):300. doi: 10.1007/s00299-024-03388-4. Plant Cell Rep. 2024. PMID: 39627595 Free PMC article.
References
-
- Agulló-Antón MA, Sanchez-Bravo J, Acosta M, Druege U. 2011. Auxins or sugars: what makes the difference in the adventitious rooting of stored carnation cuttings? Journal of Plant Growth Regulation 30: 100–113.
-
- Agulló-Antón MA, Ferrandez-Ayela A, Fernandez-Garcia N, et al. . 2014. Early steps of adventitious rooting: morphology, hormonal profiling and carbohydrate turnover in carnation stem cuttings. Physiologia Plantarum 150: 446–462. - PubMed
-
- Ahkami AH, Lischewski S, Haensch K-T, et al. . 2009. Molecular physiology of adventitious root formation in Petunia hybrida cuttings: involvement of wound response and primary metabolism. New Phytologist 181: 613–625. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

