Abnormal effector and regulatory T cell subsets in paediatric-onset multiple sclerosis
- PMID: 30759186
- PMCID: PMC6391601
- DOI: 10.1093/brain/awz017
Abnormal effector and regulatory T cell subsets in paediatric-onset multiple sclerosis
Abstract
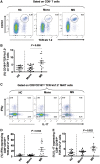
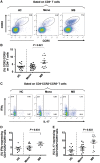
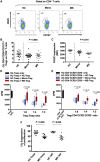
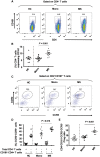
Elucidation of distinct T-cell subsets involved in multiple sclerosis immune-pathophysiology continues to be of considerable interest since an ultimate goal is to more selectively target the aberrant immune response operating in individual patients. While abnormalities of both effector (Teff) and regulatory (Treg) T cells have been reported in patients with multiple sclerosis, prior studies have mostly assessed average abnormalities in either limb of the immune response, rather than both at the same time, which limits the ability to evaluate the balance between effectors and regulators operating in the same patient. Assessing both phenotypic and functional responses of Teffs and Tregs has also proven important. In studies of adults with multiple sclerosis, in whom biological disease onset likely started many years prior to the immune assessments, an added challenge for any reported abnormality is whether the abnormality indeed contributes to the disease (and hence of interest to target therapeutically) or merely develops consequent to inflammatory injury (in which case efforts to develop targeted therapies are unlikely to be beneficial). Paediatric-onset multiple sclerosis, though rare, offers a unique window into early disease mechanisms. Here, we carried out a comprehensive integrated study, simultaneously assessing phenotype and functional responses of both effector and regulatory T cells in the same children with multiple sclerosis, monophasic inflammatory CNS disorders, and healthy controls, recruited as part of the multicentre prospective Canadian Pediatric Demyelinating Disease Study (CPDDS). Stringent standard operating procedures were developed and uniformly applied to procure, process and subsequently analyse peripheral blood cells using rigorously applied multi-parametric flow cytometry panels and miniaturized functional assays validated for use with cryopreserved cells. We found abnormally increased frequencies and exaggerated pro-inflammatory responses of CD8+CD161highTCR-Vα7.2+ MAIT T cells and CD4+CCR2+CCR5+ Teffs in paediatric-onset multiple sclerosis, compared to both control groups. CD4+CD25hiCD127lowFOXP3+ Tregs of children with multiple sclerosis exhibited deficient suppressive capacity, including diminished capacity to suppress disease-implicated Teffs. In turn, the implicated Teffs of multiple sclerosis patients were relatively resistant to suppression by normal Tregs. An abnormal Teff/Treg ratio at the individual child level best distinguished multiple sclerosis children from controls. We implicate abnormalities in both frequencies and functional responses of distinct pro-inflammatory CD4 and CD8 T cell subsets, as well as Treg function, in paediatric-onset multiple sclerosis, and suggest that mechanisms contributing to early multiple sclerosis development differ across individuals, reflecting an excess abnormality in either Teff or Treg limbs of the T cell response, or a combination of lesser abnormalities in both limbs.
Keywords: CD4+CCR2+CCR5+ T cells; CD8+ MAIT cells; multiple sclerosis; paediatric-onset multiple sclerosis; regulatory T cells.
© The Author(s) (2019). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

Comment in
-
Immune dysbalance in childhood multiple sclerosis: a 'chicken or the egg' conundrum.Brain. 2019 Mar 1;142(3):490-492. doi: 10.1093/brain/awz008. Brain. 2019. PMID: 30810210 No abstract available.
References
-
- Abrahamsson SV, Angelini DF, Dubinsky AN, Morel E, Oh U, Jones JL, et al.Non-myeloablative autologous haematopoietic stem cell transplantation expands regulatory cells and depletes IL-17 producing mucosal-associated invariant T cells in multiple sclerosis. Brain 2013; 136 (Pt 9): 2888–903. - PMC - PubMed
-
- Annibali V, Ristori G, Angelini DF, Serafini B, Mechelli R, Cannoni S, et al.CD161(high)CD8+T cells bear pathogenetic potential in multiple sclerosis. Brain 2011; 134 (Pt 2): 542–54. - PubMed
-
- Balint B, Haas J, Schwarz A, Jarius S, Furwentsches A, Engelhardt K, et al.T-cell homeostasis in pediatric multiple sclerosis: old cells in young patients. Neurology 2013; 81: 784–92. - PubMed
-
- Banwell B, Bar-Or A, Arnold DL, Sadovnick D, Narayanan S, McGowan M, et al.Clinical, environmental, and genetic determinants of multiple sclerosis in children with acute demyelination: a prospective national cohort study. Lancet Neurol 2011; 10: 436–45. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

