Aquablation of the prostate for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia
- PMID: 30759311
- PMCID: PMC6373984
- DOI: 10.1002/14651858.CD013143.pub2
Aquablation of the prostate for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia
Abstract
Background: New, minimally invasive surgeries have emerged as alternatives to transurethral resection of the prostate (TURP) for the management of lower urinary tract symptoms (LUTS) in men with benign prostatic hyperplasia (BPH). Aquablation is a novel, minimally invasive, water-based therapy, combining image guidance and robotics for the removal of prostatic tissue.
Objectives: To assess the effects of Aquablation for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia.
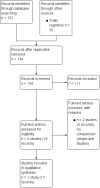
Search methods: We performed a comprehensive search using multiple databases (the Cochrane Library, MEDLINE, Embase, Scopus, Web of Science, and LILACS), trials registries, other sources of grey literature, and conference proceedings published up to 11 February 2019, with no restrictions on the language or status of publication.
Selection criteria: We included parallel-group randomised controlled trials (RCTs) and cluster-RCTs, as well as non-randomised observational prospective studies with concurrent comparison groups in which participants with BPH who underwent Aquablation.
Data collection and analysis: Two review authors independently assessed studies for inclusion at each stage, and undertook data extraction and 'Risk of bias' and GRADE assessments of the certainty of the evidence. We considered review outcomes measured up to and including 12 months after randomisation as short-term and beyond 12 months as long-term.
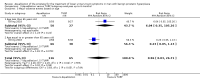
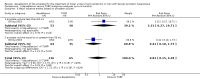
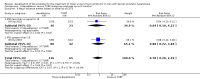
Main results: We included one RCT with 184 participants comparing Aquablation to TURP. The mean age and International Prostate Symptom Score were 65.9 years and 22.6, respectively. The mean prostate volume was 53.2 mL. We only found short-term data for all outcomes based on a single randomised trial.Primary outcomesUp to 12 months, Aquablation likely results in a similar improvement in urologic symptom scores to TURP (mean difference (MD) -0.06, 95% confidence interval (CI) -2.51 to 2.39; participants = 174; moderate-certainty evidence). We downgraded the evidence certainty by one level due to study limitations. Aquablation may also result in similar quality of life when compared to TURP (MD 0.27, 95% CI -0.24 to 0.78; participants = 174, low-certainty evidence). We downgraded the evidence certainty by two levels due to study limitations and imprecision. Aquablation may result in little to no difference in major adverse events (risk ratio (RR) 0.84, 95% CI 0.31 to 2.26; participants = 181, very low-certainty evidence) but we are very uncertain of this finding. This would correspond to 15 fewer major adverse events per 1000 participants (95% CI 64 fewer to 116 more). We downgraded the evidence certainty by one level for study limitations and two levels for imprecision.Secondary outcomesUp to 12 months, Aquablation may result in little to no difference in retreatments (RR 1.68, 95% CI 0.18 to 15.83; participants = 181, very low-certainty evidence) but we are very uncertain of this finding. This would correspond to 10 more retreatments per 1000 participants (95% CI 13 fewer to 228 more). We downgraded the evidence certainty by one level due to study limitations and two levels for imprecision.Aquablation may result in little to no difference in erectile function as measured by International Index of Erectile Function questionnaire Erectile Function domain compared to TURP (MD 2.31, 95% CI -0.63 to 5.25; participants = 64, very low-certainty evidence), and may cause slightly less ejaculatory dysfunction than TURP, as measured by Male Sexual Health Questionnaire for Ejaculatory Dysfunction (MD 2.57, 95% CI 0.60 to 4.53; participants = 121, very low-certainty evidence). However, we are very uncertain of both findings. We downgraded the evidence certainty by two levels due to study limitations and one level for imprecision for both outcomes.We did not find other prospective, comparative studies comparing Aquablation to TURP or other procedures such as laser ablation, enucleation, or other minimally invasive therapies.
Authors' conclusions: Based on short-term (up to 12 months) follow-up, the effect of Aquablation on urological symptoms is probably similar to that of TURP (moderate-certainty evidence). The effect on quality of life may also be similar (low-certainty evidence). We are very uncertain whether patients undergoing Aquablation are at higher or lower risk for major adverse events (very low-certainty evidence). We are very uncertain whether Aquablation may result in little to no difference in erectile function but offer a small improvement in preservation of ejaculatory function (both very low-certainty evidence). These conclusions are based on a single study of men with a prostate volume up to 80 mL in size. Longer-term data and comparisons with other modalities appear critical to a more thorough assessment of the role of Aquablation for the treatment of LUTS in men with BPH.
Conflict of interest statement
ECH: none known JHJ: none known MB: Boston Scientific (consultant for endourology and stone management), Auris Health (consultant for robotic surgery and endourology). MHK: none known PD: serves as Co‐ordinating Editor of Cochrane Urology. However, he was not involved in the editorial processing or decision‐making for this review. Other editors of Cochrane Urology managed the editorial process, including final sign‐off for this review.
Figures



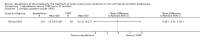
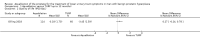
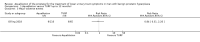





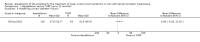
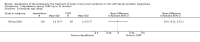
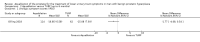
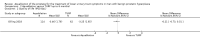






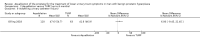
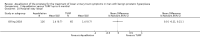






Update of
- doi: 10.1002/14651858.CD013143
References
References to studies included in this review
Gilling 2018 {published data only}
-
- A prospective multicenter randomized blinded study comparing Aquablation of the prostate with the AQUABEAM system and TURP for the treatment of LUTS. apps.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00010848.
-
- Waterjet ablation therapy for endoscopic resection of prostate tissue (WATER). clinicaltrials.gov/ct2/show/NCT02505919.
-
- Anderson P. The WATER study clinical results: a subgroup analysis of larger prostates from the phase 3, blinded, randomized trial of Aquablation vs. transurethral resection of the prostate. Canadian Urological Association Journal = Journal de l'Association des Urologues du Canada 2018;12(6Suppl2):S58. [PUBMED: 29877793]
-
- Barber N. The water study sexual function results‐a phase III blinded randomized parallel group trial of Aquablation vs. transurethral resection of the prostate with blinded outcome assessment for moderate‐to‐severe LUTS in men with benign prostatic hyperplasia. Journal of Urology 2018;199(4 Supplement):e832.
-
- Barber N, Thomas A, Aho T. The WATER study clinical results‐a phase III blinded randomized trial of Aquablation vs. TURP with blinded outcome assessment for moderate‐to‐severe LUTS in men with BPH. Journal of Clinical Urology 2018;11(1_suppl):9‐94. [DOI: 10.1177/2051415818773021] - DOI
References to studies excluded from this review
Bach 2018 {published data only}
Desai 2019 {published data only}
-
- Waterjet ablation therapy for endoscopic resection of prostate tissue II (WATERII). clinicaltrials.gov/ct2/show/NCT03123250.
-
- Zorn KC, Goldenberg SL, Paterson R, So A, Elterman D, Bhojani N. Aquablation among novice users in Canada: a WATER II subpopulation analysis. Canadian Urological Association Journal = Journal de l'Association des Urologues du Canada 2018 Oct 15 [Epub ahead of print]. [DOI: 10.5489/cuaj.5501] - DOI - PMC - PubMed
Additional references
Agarwal 2014
Aljuri 2017
-
- Aljuri N, Gilling P, Roehrborn C. How I do it: balloon tamponade of prostatic fossa following Aquablation. Canadian Journal of Urology 2017;24(4):8937‐40. [PUBMED: 28832316] - PubMed
Aoun 2015
Barry 1992
-
- Barry MJ, Fowler FJ Jr, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. Journal of Urology 1992;148(5):1549‐57; discussion 1564. [PUBMED: 1279218] - PubMed
Barry 1995
-
- Barry MJ, Williford WO, Chang Y, Machi M, Jones KM, Walker‐Corkery E, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients?. Journal of Urology 1995;154(5):1770‐4. [PUBMED: 7563343] - PubMed
Barry 1997
-
- Barry MJ, Fowler FJ Jr, Bin L, Pitts JC 3rd, Harris CJ, Mulley AG Jr. The natural history of patients with benign prostatic hyperplasia as diagnosed by North American urologists. Journal of Urology 1997;157(1):10‐4; discussion 14‐5. [PUBMED: 8976204] - PubMed
Barry 2013
-
- Barry MJ, Cantor A, Roehrborn CG, CAMUS Study Group. Relationships among participant international prostate symptom score, benign prostatic hyperplasia impact index changes and global ratings of change in a trial of phytotherapy in men with lower urinary tract symptoms. Journal of Urology 2013;189(3):987‐92. [PUBMED: 23017510] - PMC - PubMed
Bhojani 2014
-
- Bhojani N, Gandaglia G, Sood A, Rai A, Pucheril D, Chang SL, et al. Morbidity and mortality after benign prostatic hyperplasia surgery: data from the American College of Surgeons national surgical quality improvement program. Journal of Endourology 2014;28(7):831‐40. [PUBMED: 24517323] - PubMed
Chapple 2017
Cornu 2010
-
- Cornu JN, Cussenot O, Haab F, Lukacs B. A widespread population study of actual medical management of lower urinary tract symptoms related to benign prostatic hyperplasia across Europe and beyond official clinical guidelines. European Urology 2010;58(3):450‐6. [PUBMED: 20554374] - PubMed
Covidence 2017 [Computer program]
-
- Veritas Health Innovation. Covidence. Version accessed 16 August 2017. Melbourne, Australia: Veritas Health Innovation, 2013.
Crawford 2006
-
- Crawford ED, Wilson SS, McConnell JD, Slawin KM, Lieber MC, Smith JA, et al. Baseline factors as predictors of clinical progression of benign prostatic hyperplasia in men treated with placebo. Journal of Urology 2006;175(4):1422‐6; discussion 1426‐7. [PUBMED: 16516013] - PubMed
Deeks 2017
-
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors),Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handboo. Available from www.training.cochrane.org/handbook.
Dindo 2004
Dunphy 2015
EAU 2017
-
- European Association of Urology. Treatment of non‐neurogenic male LUTS. uroweb.org/guideline/treatment‐of‐non‐neurogenic‐male‐luts/ (accessed 1 March 2018).
Egan 2016
-
- Egan KB. The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urologic Clinics of North America 2016;43(3):289‐97. [PUBMED: 27476122] - PubMed
Endnote [Computer program]
-
- Clarivate Analytics. EndNote. Version 7.5. Clarivate Analytics, 2016.
Faber 2015
-
- Faber K, Abreu AL, Ramos P, Aljuri N, Mantri S, Gill I, et al. Image‐guided robot‐assisted prostate ablation using water jet‐hydrodissection: initial study of a novel technology for benign prostatic hyperplasia. Journal of Endourology 2015;29(1):63‐9. [PUBMED: 25000418] - PubMed
Foster 2018
-
- Foster HE, Barry MJ, Dahm P, Gandhi MC, Kaplan SA, Kohler TS, et al. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA Guideline. Journal of Urology 2018;200(3):612‐9. [PUBMED: 29775639] - PubMed
Gilling 2016
-
- Gilling P, Reuther R, Kahokehr A, Fraundorfer M. Aquablation ‐ image‐guided robot‐assisted waterjet ablation of the prostate: initial clinical experience. BJU International 2016;117(6):923‐9. [PUBMED: 26477826] - PubMed
Gilling 2017
-
- Gilling P, Anderson P, Tan A. Aquablation of the prostate for symptomatic benign prostatic hyperplasia: 1‐year results. Journal of Urology 2017;197(6):1565‐72. [PUBMED: 28111300] - PubMed
GRADEpro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 13 December 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2008
Guyatt 2011a
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] - PubMed
Guyatt 2011b
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] - PubMed
Guyatt 2011c
-
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] - PubMed
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2017a
-
- Higgins JPT, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hirst 2018
-
- Hirst A, Philippou Y, Blazeby J, Campbell B, Campbell M, Feinberg J, et al. No surgical innovation without evaluation: evolution and further development of the IDEAL framework and recommendations. Annals of Surgery 2018 Apr 24 [Epub ahead of print]. [DOI: 10.1097/SLA.0000000000002794.] - DOI - PubMed
Homma 1997
-
- Homma Y, Kawabe K, Tsukamoto T, Yamanaka H, Okada K, Okajima E, et al. Epidemiologic survey of lower urinary tract symptoms in Asia and Australia using the international prostate symptom score. International Journal of Urology : Official Journal of the Japanese Urological Association 1997;4(1):40‐6. [PUBMED: 9179665] - PubMed
Jaeschke 1989
-
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clinical Trials 1989;10(4):407‐15. [PUBMED: 2691207] - PubMed
Johnston 2013
Jung 2017a
Jung 2017b
-
- Jung JH, Shin TY, McCutcheon KA, Borofsky M, Narayan V, Young S, et al. Prostatic arterial embolization for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD012867] - DOI - PMC - PubMed
Kang 2019
Kaplan 2015
-
- Kaplan AL, Agarwal N, Setlur NP, Tan HJ, Niedzwiecki D, McLaughlin N, et al. Measuring the cost of care in benign prostatic hyperplasia using time‐driven activity‐based costing (TDABC). Healthcare (Amsterdam, Netherlands) 2015;3(1):43‐8. [PUBMED: 26179588] - PubMed
Kozminski 2015
Lebdai 2018
-
- Lebdai S, Chevrot A, Doizi S, Pradere B, Delongchamps NB, Benchikh A, et al. Do patients have to choose between ejaculation and miction? A systematic review about ejaculation preservation technics for benign prostatic obstruction surgical treatment. World Journal of Urology 2018 Jul 2 [Epub ahead of print]. [DOI: 10.1007/s00345-018-2368-6] - DOI - PubMed
Lee 2018
Leissner 1979
-
- Leissner KH, Tisell LE. The weight of the human prostate. Scandinavian Journal of Urology and Nephrology 1979;13(2):137‐42. [PUBMED: 90380] - PubMed
Liberati 2009
Magistro 2017
-
- Magistro G, Chapple CR, Elhilali M, Gilling P, McVary KT, Roehrborn CG, et al. Emerging minimally invasive treatment options for male lower urinary tract symptoms. European Urology 2017;72(6):986‐97. [PUBMED: 28734706] - PubMed
Mamoulakis 2014
-
- Mamoulakis C, Sofras F, Rosette J, Omar MI, Lam TBL, N'Dow JM, et al. Bipolar versus monopolar transurethral resection of the prostate for lower urinary tract symptoms secondary to benign prostatic obstruction. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD009629.pub3] - DOI - PMC - PubMed
Martin 2014
-
- Martin S, Lange K, Haren MT, Taylor AW, Wittert G. Risk factors for progression or improvement of lower urinary tract symptoms in a prospective cohort of men. Journal of Urology 2014;191(1):130‐7. [PUBMED: 23770136] - PubMed
McNicholas 2016
McVary 2011
-
- McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. Journal of Urology 2011;185(5):1793‐803. [PUBMED: 21420124] - PubMed
Nagele 2011
-
- Nagele U, Kugler M, Nicklas A, Merseburger AS, Walcher U, Mikuz G, et al. Waterjet hydrodissection: first experiences and short‐term outcomes of a novel approach to bladder tumor resection. World Journal of Urology 2011;29(4):423‐7. [PUBMED: 21305303] - PubMed
NICE guidance
-
- Transurethral water jet ablation for lower urinary tract symptoms caused by benign prostatic hyperplasia. https://www.nice.org.uk/guidance/ipg629 (accessed 11 February 2019). - PubMed
Omar 2014
-
- Omar MI, Lam TB, Alexander CE, Graham J, Mamoulakis C, Imamura M, et al. Systematic review and meta‐analysis of the clinical effectiveness of bipolar compared with monopolar transurethral resection of the prostate (TURP). BJU International 2014;113(1):24‐35. [PUBMED: 24053602] - PubMed
Pariser 2015
-
- Pariser JJ, Pearce SM, Patel SG, Bales GT. National trends of simple prostatectomy for benign prostatic hyperplasia with an analysis of risk factors for adverse perioperative outcomes. Urology 2015;86(4):721‐5. [PUBMED: 26276574] - PubMed
PROCEPT BioRobotics
-
- PROCEPT BioRobotics, Redwood Shores, California, USA. www.procept‐biorobotics.com.
Rees 2015
-
- Rees J. Patients not P values. BJU International 2015; Vol. 115, issue 5:678‐9. [PUBMED: 25885560] - PubMed
Reich 2008
-
- Reich O, Gratzke C, Bachmann A, Seitz M, Schlenker B, Hermanek P, et al. Morbidity, mortality and early outcome of transurethral resection of the prostate: a prospective multicenter evaluation of 10,654 patients. Journal of Urology 2008;180(1):246‐9. [PUBMED: 18499179] - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rieken 2018
-
- Rieken M, Antunes‐Lopes T, Geavlete B, Marcelissen T. What is new with sexual side effects after transurethral male lower urinary tract symptom surgery?. European Urology Focus 2018;4(1):43‐5. [PUBMED: 29803559] - PubMed
Roehrborn 2008a
-
- Roehrborn CG. Pathology of benign prostatic hyperplasia. International Journal of Impotence Research 2008;20 Suppl 3:S11‐8. [PUBMED: 19002119] - PubMed
Roehrborn 2008b
Rosen 1997
-
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49(6):822‐30. [PUBMED: 9187685] - PubMed
Rosen 2004
-
- Rosen RC, Catania J, Pollack L, Althof S, O'Leary M, Seftel AD. Male Sexual Health Questionnaire (MSHQ): scale development and psychometric validation. Urology 2004;64(4):777‐82. [PUBMED: 15491719] - PubMed
Rosen 2007
-
- Rosen RC, Catania JA, Althof SE, Pollack LM, O'Leary M, Seftel AD, et al. Development and validation of four‐item version of Male Sexual Health Questionnaire to assess ejaculatory dysfunction. Urology 2007;69(5):805‐9. [PUBMED: 17482908] - PubMed
Rosen 2011
-
- Rosen RC, Allen KR, Ni X, Araujo AB. Minimal clinically important differences in the erectile function domain of the International Index of Erectile Function scale. European Urology 2011;60(5):1010‐6. [PUBMED: 21855209] - PubMed
Russo 2015
-
- Russo GI, Castelli T, Urzi D, Privitera S, Vignera S, Condorelli RA, et al. Emerging links between non‐neurogenic lower urinary tract symptoms secondary to benign prostatic obstruction, metabolic syndrome and its components: a systematic review. International Journal of Urology : Official Journal of the Japanese Urological Association 2015;22(11):982‐90. [PUBMED: 26193757] - PubMed
Schünemann 2013
-
- Schünemann HJ, Tugwell P, Reeves BC, Akl EA, Santesso N, Spencer FA, et al. Non‐randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Research Synthesis Methods 2013;4(1):49‐62. [PUBMED: 26053539] - PubMed
Schünemann 2017a
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Schünemann 2017b
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Spaliviero 2010
-
- Spaliviero M, Strom KH, Gu X, Araki M, Culkin DJ, Wong C. Does Greenlight HPS(™) laser photoselective vaporization prostatectomy affect sexual function?. Journal of Endourology 2010;24(12):2051‐7. [PUBMED: 20964486] - PubMed
Sterne 2016a
Sterne 2016b
-
- Sterne JA, Higgins JP, Elbers RG, Reeves BC and the development group for ROBINS‐I. Risk of bias in non‐randomized studies of interventions (ROBINS‐I): detailed guidance, updated 12 October 2016. Available from www.riskofbias.info (accessed 6 July 2018).
Sterne 2017
-
- Sterne JA, Egger M, Moher D, Boutron I (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J,Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Taktak 2018
WHO 2012
-
- World Health Organization. Proposed working definition of an older person in Africa for the MDS project. www.who.int/healthinfo/survey/ageingdefnolder/en (accessed 1 March 2018).
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

