Electrobiofabrication: electrically based fabrication with biologically derived materials
- PMID: 30759423
- PMCID: PMC7025432
- DOI: 10.1088/1758-5090/ab06ea
Electrobiofabrication: electrically based fabrication with biologically derived materials
Abstract
While conventional material fabrication methods focus on form and strength to achieve function, the fabrication of material systems for emerging life science applications will need to satisfy a more subtle set of requirements. A common goal for biofabrication is to recapitulate complex biological contexts (e.g. tissue) for applications that range from animal-on-a-chip to regenerative medicine. In these cases, the material systems will need to: (i) present appropriate surface functionalities over a hierarchy of length scales (e.g. molecular features that enable cell adhesion and topographical features that guide differentiation); (ii) provide a suite of mechanobiological cues that promote the emergence of native-like tissue form and function; and (iii) organize structure to control cellular ingress and molecular transport, to enable the development of an interconnected cellular community that is engaged in cell signaling. And these requirements are not likely to be static but will vary over time and space, which will require capabilities of the material systems to dynamically respond, adapt, heal and reconfigure. Here, we review recent advances in the use of electrically based fabrication methods to build material systems from biological macromolecules (e.g. chitosan, alginate, collagen and silk). Electrical signals are especially convenient for fabrication because they can be controllably imposed to promote the electrophoresis, alignment, self-assembly and functionalization of macromolecules to generate hierarchically organized material systems. Importantly, this electrically based fabrication with biologically derived materials (i.e. electrobiofabrication) is complementary to existing methods (photolithographic and printing), and enables access to the biotechnology toolbox (e.g. enzymatic-assembly and protein engineering, and gene expression) to offer exquisite control of structure and function. We envision that electrobiofabrication will emerge as an important platform technology for organizing soft matter into dynamic material systems that mimic biology's complexity of structure and versatility of function.
Figures

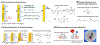
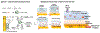

References
-
- Groll J. et al. Biofabrication: reappraising the definition of an evolving field. Biofabrication. 2016;8:013001. - PubMed
-
- Gordonov T, Kim E, Cheng Y, Ben-Yoav H, Ghodssi R, Rubloff G, Yin J-J, Payne GF and Bentley WE 2014. Electronic modulation of biochemical signal generation Nat. Nanotechnol 9 605–10 - PubMed
-
- Wang H, Ma X and Hao Y 2017. Electronic devices for human-machine interfaces Adv. Mater. Interfaces 4 1–20
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
