Targeting Immune Signaling Checkpoints in Acute Myeloid Leukemia
- PMID: 30759726
- PMCID: PMC6406869
- DOI: 10.3390/jcm8020236
Targeting Immune Signaling Checkpoints in Acute Myeloid Leukemia
Abstract
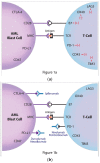
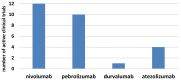
The modest successes of targeted therapies along with the curative effects of allogeneic hematopoietic stem cell transplantation (alloHSCT) in acute myeloid leukemia (AML) stimulate the development of new immunotherapies. One of the promising methods of immunotherapy is the activation of immune response by the targeting of negative control checkpoints. The two best-known inhibitory immune checkpoints are cytotoxic T-lymphocyte antigen-4 (CTLA-4) and the programmed cell death protein 1 receptor (PD-1). In AML, PD-1 expression is observed in T-cell subpopulations, including T regulatory lymphocytes. Increased PD-1 expression on CD8+ T lymphocytes may be one of the factors leading to dysfunction of cytotoxic T cells and inhibition of the immune response during the progressive course of AML. Upregulation of checkpoint molecules was observed after alloHSCT and therapy with hypomethylating agents, pointing to a potential clinical application in these settings. Encouraging results from recent clinical trials (a response rate above 50% in a relapsed setting) justify further clinical use. The most common clinical trials employ two PD-1 inhibitors (nivolumab and pembrolizumab) and two anti-PD-L1 (programmed death-ligand 1) monoclonal antibodies (atezolizumab and durvalumab). Several other inhibitors are under development or in early phases of clinical trials. The results of these clinical trials are awaited with great interest in, as they may allow for the established use of checkpoint inhibitors in the treatment of AML.
Keywords: acute myeloid leukemia; immunotherapy; programmed cell death protein 1 receptor/ programmed death-ligand 1 (PD-1/PD-L1) signaling.
Conflict of interest statement
The author declares no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

