Preclinical Evaluation of Long-Term Neuroprotective Effects of BDNF-Engineered Mesenchymal Stromal Cells as Intravitreal Therapy for Chronic Retinal Degeneration in Rd6 Mutant Mice
- PMID: 30759764
- PMCID: PMC6387230
- DOI: 10.3390/ijms20030777
Preclinical Evaluation of Long-Term Neuroprotective Effects of BDNF-Engineered Mesenchymal Stromal Cells as Intravitreal Therapy for Chronic Retinal Degeneration in Rd6 Mutant Mice
Abstract
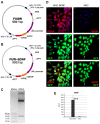
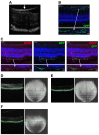
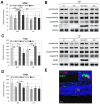
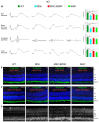
This study aimed to investigate whether the transplantation of genetically engineered bone marrow-derived mesenchymal stromal cells (MSCs) to overexpress brain-derived neurotrophic factor (BDNF) could rescue the chronic degenerative process of slow retinal degeneration in the rd6 (retinal degeneration 6) mouse model and sought to identify the potential underlying mechanisms. Rd6 mice were subjected to the intravitreal injection of lentivirally modified MSC-BDNF or unmodified MSC or saline. In vivo morphology, electrophysiological retinal function (ERG), and the expression of apoptosis-related genes, as well as BDNF and its receptor (TrkB), were assessed in retinas collected at 28 days and three months after transplantation. We observed that cells survived for at least three months after transplantation. MSC-BDNF preferentially integrated into the outer retinal layers and considerably rescued damaged retinal cells, as evaluated by ERG and immunofluorescence staining. Additionally, compared with controls, the therapy with MSC-BDNF was associated with the induction of molecular changes related to anti-apoptotic signaling. In conclusion, BDNF overexpression observed in retinas after MSC-BDNF treatment could enhance the neuroprotective properties of transplanted autologous MSCs alone in the chronically degenerated retina. This research provides evidence for the long-term efficacy of genetically-modified MSC and may represent a strategy for treating various forms of degenerative retinopathies in the future.
Keywords: BDNF; MSC; OCT; lentiviral vectors; rd6; regenerative medicine; retinal degeneration; tissue imaging.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

