Differential Response Following Infection of Mouse CNS with Virulent and Attenuated Vaccinia Virus Strains
- PMID: 30759813
- PMCID: PMC6466266
- DOI: 10.3390/vaccines7010019
Differential Response Following Infection of Mouse CNS with Virulent and Attenuated Vaccinia Virus Strains
Abstract
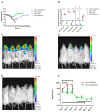
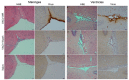
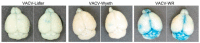
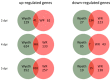
Viral infections of the central nervous system (CNS) lead to a broad range of pathologies. CNS infections with Orthopox viruses have been mainly documented as an adverse reaction to smallpox vaccination with vaccinia virus. To date, there is insufficient data regarding the mechanisms underlying pathological viral replication or viral clearance. Therefore, informed risk assessment of vaccine adverse reactions or outcome prediction is limited. This work applied a model of viral infection of the CNS, comparing neurovirulent with attenuated strains. We followed various parameters along the disease and correlated viral load, morbidity, and mortality with tissue integrity, innate and adaptive immune response and functionality of the blood⁻brain barrier. Combining these data with whole brain RNA-seq analysis performed at different time points indicated that neurovirulence is associated with host immune silencing followed by induction of tissue damage-specific pathways. In contrast, brain infection with attenuated strains resulted in rapid and robust induction of innate and adaptive protective immunity, followed by viral clearance and recovery. This study significantly improves our understanding of the mechanisms and processes determining the consequence of viral CNS infection and highlights potential biomarkers associated with such outcomes.
Keywords: RNA-seq; brain; meningoencephalitis; neurovirulence; smallpox; vaccinia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Vaccinia virus-induced smallpox postvaccinal encephalitis in case of blood-brain barrier damage.Vaccine. 2012 Feb 8;30(7):1397-405. doi: 10.1016/j.vaccine.2011.08.116. Epub 2012 Jan 9. Vaccine. 2012. PMID: 22227123
-
Smallpox vaccination and adverse reactions. Guidance for clinicians.MMWR Recomm Rep. 2003 Feb 21;52(RR-4):1-28. MMWR Recomm Rep. 2003. PMID: 12617510
-
The Virulence of Different Vaccinia Virus Strains Is Directly Proportional to Their Ability To Downmodulate Specific Cell-Mediated Immune Compartments In Vivo.J Virol. 2019 Mar 5;93(6):e02191-18. doi: 10.1128/JVI.02191-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30567985 Free PMC article.
-
The role of T-cell-mediated mechanisms in virus infections of the nervous system.Curr Top Microbiol Immunol. 2001;253:219-45. doi: 10.1007/978-3-662-10356-2_11. Curr Top Microbiol Immunol. 2001. PMID: 11417137 Review.
-
Modified Vaccinia virus Ankara: innate immune activation and induction of cellular signalling.Vaccine. 2013 Sep 6;31(39):4231-4. doi: 10.1016/j.vaccine.2013.03.017. Epub 2013 Mar 21. Vaccine. 2013. PMID: 23523404 Review.
Cited by
-
A comprehensive preclinical study supporting clinical trial of oncolytic chimeric poxvirus CF33-hNIS-anti-PD-L1 to treat breast cancer.Mol Ther Methods Clin Dev. 2021 Dec 6;24:102-116. doi: 10.1016/j.omtm.2021.12.002. eCollection 2022 Mar 10. Mol Ther Methods Clin Dev. 2021. PMID: 35024377 Free PMC article.
-
Preliminary nonclinical safety and immunogenicity of an rVSV-ΔG-SARS-CoV-2-S vaccine in mice, hamsters, rabbits and pigs.Arch Toxicol. 2022 Mar;96(3):859-875. doi: 10.1007/s00204-021-03214-w. Epub 2022 Jan 15. Arch Toxicol. 2022. PMID: 35032184 Free PMC article.
-
Induction of Innate Immune Response by TLR3 Agonist Protects Mice against SARS-CoV-2 Infection.Viruses. 2022 Jan 19;14(2):189. doi: 10.3390/v14020189. Viruses. 2022. PMID: 35215785 Free PMC article.
-
Interferon α/β Decoy Receptor Encoded by a Variant in the Dryvax Smallpox Vaccine Contributes to Virulence and Correlates with Severe Vaccine Side Effects.mBio. 2022 Feb 22;13(1):e0010222. doi: 10.1128/mbio.00102-22. Epub 2022 Feb 22. mBio. 2022. PMID: 35189701 Free PMC article.
-
Increased lethality in influenza and SARS-CoV-2 coinfection is prevented by influenza immunity but not SARS-CoV-2 immunity.Nat Commun. 2021 Oct 5;12(1):5819. doi: 10.1038/s41467-021-26113-1. Nat Commun. 2021. PMID: 34611155 Free PMC article.
References
-
- Fenner F., Henderson D.A., Arita I., Jezek Z., Ladnyi I.D. Smallpox and its Eradication. In: WHO, editor. History of International Public Health. World Health Organization; Geneva Switzerland: 1988. pp. 1371–1409. No. 6.
-
- Casey C.G., Iskander J.K., Roper M.H., Mast E.E., Wen X.J., Torok T.J., Chapman L.E., Swerdlow D.L., Morgan J., Heffelfinger J.D., et al. Adverse events associated with smallpox vaccination in the United States, January-October 2003. JAMA. 2005;294:2734–2743. doi: 10.1001/jama.294.21.2734. - DOI - PubMed
LinkOut - more resources
Full Text Sources

