Clonal Hematopoiesis with Oncogenic Potential (CHOP): Separation from CHIP and Roads to AML
- PMID: 30759825
- PMCID: PMC6387423
- DOI: 10.3390/ijms20030789
Clonal Hematopoiesis with Oncogenic Potential (CHOP): Separation from CHIP and Roads to AML
Abstract
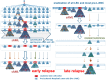
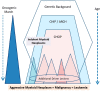
The development of leukemia is a step-wise process that is associated with molecular diversification and clonal selection of neoplastic stem cells. Depending on the number and combinations of lesions, one or more sub-clones expand/s after a variable latency period. Initial stages may develop early in life or later in adulthood and include premalignant (indolent) stages and the malignant phase, defined by an acute leukemia. We recently proposed a cancer model in which the earliest somatic lesions are often age-related early mutations detectable in apparently healthy individuals and where additional oncogenic mutations will lead to the development of an overt neoplasm that is usually a preleukemic condition such as a myelodysplastic syndrome. These neoplasms may or may not transform to overt acute leukemia over time. Thus, depending on the type and number of somatic mutations, clonal hematopoiesis (CH) can be divided into CH with indeterminate potential (CHIP) and CH with oncogenic potential (CHOP). Whereas CHIP mutations per se usually create the molecular background of a neoplastic process, CHOP mutations are disease-related or even disease-specific lesions that trigger differentiation and/or proliferation of neoplastic cells. Over time, the acquisition of additional oncogenic events converts preleukemic neoplasms into secondary acute myeloid leukemia (sAML). In the present article, recent developments in the field are discussed with a focus on CHOP mutations that lead to distinct myeloid neoplasms, their role in disease evolution, and the impact of additional lesions that can drive a preleukemic neoplasm into sAML.
Keywords: cancer; clonal evolution; neoplastic stem cells; premalignant states.
Conflict of interest statement
The authors declare that they have no conflict to disclose in this study.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

