Molecular Mechanisms of Colistin-Induced Nephrotoxicity
- PMID: 30759858
- PMCID: PMC6384669
- DOI: 10.3390/molecules24030653
Molecular Mechanisms of Colistin-Induced Nephrotoxicity
Abstract
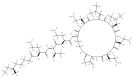
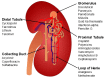
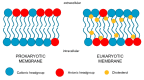
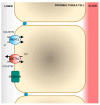
The emergence of multidrug resistant (MDR) infections and the shortage of new therapeutic options have made colistin, a polymyxin antibiotic, the main option for the treatment of MDR Gram-negative bacterial infections in the last decade. However, the rapid onset of renal damage often prevents the achievement of optimal therapeutic doses and/or forces the physicians to interrupt the therapy, increasing the risk of drug resistance. The proper management of colistin-induced nephrotoxicity remains challenging, mostly because the investigation of the cellular and molecular pharmacology of this drug, off the market for decades, has been largely neglected. For years, the renal damage induced by colistin was considered a mere consequence of the detergent activity of this drug on the cell membrane of proximal tubule cells. Lately, it has been proposed that the intracellular accumulation is a precondition for colistin-mediated renal damage, and that mitochondria might be a primary site of damage. Antioxidant approaches (e.g., ascorbic acid) have shown promising results in protecting the kidney of rodents exposed to colistin, yet none of these strategies have yet reached the bedside. Here we provide a critical overview of the possible mechanisms that may contribute to colistin-induced renal damage and the potential protective strategies under investigation.
Keywords: acute kidney injury; colistin; mitochondria; nephrotoxicity; polymyxins; proximal tubule.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
[Polymyxins - review with emphasis on nephrotoxicity].Rev Assoc Med Bras (1992). 2009 Nov-Dec;55(6):752-9. doi: 10.1590/s0104-42302009000600023. Rev Assoc Med Bras (1992). 2009. PMID: 20191233 Review. Portuguese.
-
Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections.Clin Infect Dis. 2005 May 1;40(9):1333-41. doi: 10.1086/429323. Epub 2005 Mar 22. Clin Infect Dis. 2005. PMID: 15825037 Review.
-
Efficacy and safety of low-dose colistin in the treatment for infections caused by multidrug-resistant gram-negative bacteria.J Clin Pharm Ther. 2014 Jun;39(3):272-6. doi: 10.1111/jcpt.12138. Epub 2014 Mar 5. J Clin Pharm Ther. 2014. PMID: 24593154
-
Pharmacokinetics/pharmacodynamics of colistin and polymyxin B: are we there yet?Int J Antimicrob Agents. 2016 Dec;48(6):592-597. doi: 10.1016/j.ijantimicag.2016.09.010. Epub 2016 Oct 18. Int J Antimicrob Agents. 2016. PMID: 27793510 Free PMC article. Review.
-
L-carnitine co-administration prevents colistin-induced mitochondrial permeability transition and reduces the risk of acute kidney injury in mice.Sci Rep. 2024 Jul 16;14(1):16444. doi: 10.1038/s41598-024-67171-x. Sci Rep. 2024. PMID: 39013979 Free PMC article.
Cited by
-
Omeprazole Prevents Colistin-Induced Nephrotoxicity in Rats: Emphasis on Oxidative Stress, Inflammation, Apoptosis and Colistin Accumulation in Kidneys.Pharmaceuticals (Basel). 2022 Jun 23;15(7):782. doi: 10.3390/ph15070782. Pharmaceuticals (Basel). 2022. PMID: 35890080 Free PMC article.
-
Combining SNAPs with antibiotics shows enhanced synergistic efficacy against S. aureus and P. aeruginosa biofilms.NPJ Biofilms Microbiomes. 2023 Jun 8;9(1):36. doi: 10.1038/s41522-023-00401-8. NPJ Biofilms Microbiomes. 2023. PMID: 37291132 Free PMC article.
-
Neurotoxicity associated with colistin methanesulfonate treatment is enhanced by concomitant sevoflurane inhalation.Toxicol Rep. 2022 Jun 2;9:1255-1260. doi: 10.1016/j.toxrep.2022.05.020. eCollection 2022. Toxicol Rep. 2022. PMID: 36518416 Free PMC article.
-
Synergistic Activity of Tetrandrine and Colistin against mcr-1-Harboring Escherichia coli.Antibiotics (Basel). 2022 Oct 2;11(10):1346. doi: 10.3390/antibiotics11101346. Antibiotics (Basel). 2022. PMID: 36290004 Free PMC article.
-
Pharmacokinetics of Antibacterial Agents in the Elderly: The Body of Evidence.Biomedicines. 2023 Jun 4;11(6):1633. doi: 10.3390/biomedicines11061633. Biomedicines. 2023. PMID: 37371728 Free PMC article. Review.
References
-
- Koyama Y. A new antibiotic “colistin” produced by spore-forming soil bacteria. J. Antibiot. 1950;3:457–458.
-
- Kirby W.M.M., Evans Roberts C., Jr. Colistin. JAMA. 1961;177:854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

