A Randomized Feeding Trial of Iron-Biofortified Beans on School Children in Mexico
- PMID: 30759887
- PMCID: PMC6412428
- DOI: 10.3390/nu11020381
A Randomized Feeding Trial of Iron-Biofortified Beans on School Children in Mexico
Abstract
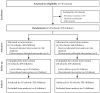
Iron deficiency is a major public health problem worldwide, with the highest burden among children. The objective of this randomized efficacy feeding trial was to determine the effects of consuming iron-biofortified beans (Fe-Beans) on the iron status in children, compared to control beans (Control-Beans). A cluster-randomized trial of biofortified beans (Phaseolus vulgaris L), bred to enhance iron content, was conducted over 6 months. The participants were school-aged children (n = 574; 5⁻12 years), attending 20 rural public boarding schools in the Mexican state of Oaxaca. Double-blind randomization was conducted at the school level; 20 schools were randomized to receive either Fe-Beans (n = 10 schools, n = 304 students) or Control-Beans (n = 10 schools, n = 366 students). School administrators, children, and research and laboratory staff were blinded to the intervention group. Iron status (hemoglobin (Hb), serum ferritin (SF), soluble transferrin receptor (sTfR), total body iron (TBI), inflammatory biomarkers C-reactive protein (CRP) and -1-acid glycoprotein (AGP)), and anthropometric indices for individuals were evaluated at the enrollment and at the end of the trial. The hemoglobin concentrations were adjusted for altitude, and anemia was defined in accordance with age-specific World Health Organization (WHO) criteria (i.e., Hb <115 g/L for <12 years and Hb <120 g/L for 12 years). Serum ferritin concentrations were adjusted for inflammation using BRINDA methods, and iron deficiency was defined as serum ferritin at less than 15.0 µg/L. Total body iron was calculated using Cook's equation. Mixed models were used to examine the effects of Fe-Beans on hematological outcomes, compared to Control-Beans, adjusting for the baseline indicator, with school as a random effect. An analysis was conducted in 10 schools (n = 269 students) in the Fe-Beans group and in 10 schools (n = 305 students) in the Control-Beans group that completed the follow-up. At baseline, 17.8% of the children were anemic and 11.3% were iron deficient (15.9%, BRINDA-adjusted). A total of 6.3% of children had elevated CRP (>5.0 mg/L), and 11.6% had elevated AGP (>1.0 g/L) concentrations at baseline. During the 104 days when feeding was monitored, the total mean individual iron intake from the study beans (Fe-bean group) was 504 mg (IQR: 352, 616) over 68 mean feeding days, and 295 mg (IQR: 197, 341) over 67 mean feeding days in the control group (p < 0.01). During the cluster-randomized efficacy trial, indicators of iron status, including hemoglobin, serum ferritin, soluble transferrin receptor, and total body iron concentrations improved from the baseline to endline (6 months) in both the intervention and control groups. However, Fe-Beans did not significantly improve the iron status indicators, compared to Control-Beans. Similarly, there were no significant effects of Fe-Beans on dichotomous outcomes, including anemia and iron deficiency, compared to Control-Beans. In this 6-month cluster-randomized efficacy trial of iron-biofortified beans in school children in Mexico, indicators of iron status improved in both the intervention and control groups. However, there were no significant effects of Fe-Beans on iron biomarkers, compared to Control-Beans. This trial was registered at clinicaltrials.gov as NCT03835377.
Keywords: Mexico; anemia; beans; biofortification; children; international nutrition; iron.
Conflict of interest statement
J.D.H. received funding as an expert consultant for HarvestPlus. S.M. is an unpaid board member for a diagnostic start up focused on measurement of nutritional biomarkers at the point-of-care utilizing the results from his research. The other authors have no conflict of interest to disclose. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; or in the writing of the manuscript.
Figures
Similar articles
-
Consuming Iron Biofortified Beans Increases Iron Status in Rwandan Women after 128 Days in a Randomized Controlled Feeding Trial.J Nutr. 2016 Aug;146(8):1586-92. doi: 10.3945/jn.115.224741. Epub 2016 Jun 29. J Nutr. 2016. PMID: 27358417 Clinical Trial.
-
A Randomized Trial of Iron-Biofortified Pearl Millet in School Children in India.J Nutr. 2015 Jul;145(7):1576-81. doi: 10.3945/jn.114.208009. Epub 2015 May 6. J Nutr. 2015. PMID: 25948782 Clinical Trial.
-
Increased Iron Status during a Feeding Trial of Iron-Biofortified Beans Increases Physical Work Efficiency in Rwandan Women.J Nutr. 2020 May 1;150(5):1093-1099. doi: 10.1093/jn/nxaa016. J Nutr. 2020. PMID: 32006009 Free PMC article.
-
Iron-biofortified staple food crops for improving iron status: a review of the current evidence.Curr Opin Biotechnol. 2017 Apr;44:138-145. doi: 10.1016/j.copbio.2017.01.003. Epub 2017 Jan 25. Curr Opin Biotechnol. 2017. PMID: 28131049 Free PMC article. Review.
-
Iron biofortification interventions to improve iron status and functional outcomes.Proc Nutr Soc. 2019 May;78(2):197-207. doi: 10.1017/S0029665118002847. Epub 2019 Jan 30. Proc Nutr Soc. 2019. PMID: 30698117
Cited by
-
Can Improved Legume Varieties Optimize Iron Status in Low- and Middle-Income Countries? A Systematic Review.Adv Nutr. 2020 Sep 1;11(5):1315-1324. doi: 10.1093/advances/nmaa038. Adv Nutr. 2020. PMID: 32330226 Free PMC article.
-
Dietary Bean Consumption and Human Health.Nutrients. 2019 Dec 17;11(12):3074. doi: 10.3390/nu11123074. Nutrients. 2019. PMID: 31861044 Free PMC article.
-
Dietary Approaches to Iron Deficiency Prevention in Childhood-A Critical Public Health Issue.Nutrients. 2022 Apr 12;14(8):1604. doi: 10.3390/nu14081604. Nutrients. 2022. PMID: 35458166 Free PMC article. Review.
-
Dietary Trace Minerals.Nutrients. 2019 Nov 19;11(11):2823. doi: 10.3390/nu11112823. Nutrients. 2019. PMID: 31752257 Free PMC article.
-
Iron Bioavailability from Multiple Biofortified Foods Using an In Vitro Digestion, Caco-2 Assay for Optimizing a Cyclical Menu for a Randomized Efficacy Trial.Curr Dev Nutr. 2021 Sep 8;5(9):nzab111. doi: 10.1093/cdn/nzab111. eCollection 2021 Sep. Curr Dev Nutr. 2021. PMID: 34604692 Free PMC article.
References
-
- WHO . Iron Deficiency Anaemia Assessment, Prevention, and Control: A Guide for Programme Managers. WHO; Geneva, Switzerland: 2001.
-
- UNSCN . 4th Report—The World Nutrition Situation: Nutrition Throughout the Life Cycle. UNSCN; Rome, Italy: 2000.
-
- ACC/SCN . Fourth Report on the World Nutrition Situation. ACC/SCN in collaboration with IFPRI; Geneva, Switzerland: 2000.
-
- WHO . Micronutrient Deficiencies: Iron Deficiency Anaemia. WHO; Geneva, Switzerland: 2017.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous