Evaluation of IGF1/IGFBP3 Molar Ratio as an Effective Tool for Assessing the Safety of Growth Hormone Therapy in Small-for-gestational-age, Growth Hormone-Deficient and Prader-Willi Children
- PMID: 30759961
- PMCID: PMC6745465
- DOI: 10.4274/jcrpe.galenos.2019.2018.0277
Evaluation of IGF1/IGFBP3 Molar Ratio as an Effective Tool for Assessing the Safety of Growth Hormone Therapy in Small-for-gestational-age, Growth Hormone-Deficient and Prader-Willi Children
Abstract
Objective: IGF1 concentration is the most widely used parameter for the monitoring and therapeutic adaptation of recombinant human growth hormone (rGH) treatment. However, more than half the variation of the therapeutic response is accounted for by variability in the serum concentrations of IGF1 and IGFBP3. We therefore compared the use of IGF1/IGFBP3 molar ratio with that of IGF1 concentration alone.
Methods: We selected 92 children on rGH for this study and assigned them to three groups on the basis of growth deficiency etiology: small for gestational age (SGA), GH deficiency (GHD) and Prader-Willi syndrome (PWS). Plasma IGF1 and IGFBP3 concentrations and their molar ratio were determined.
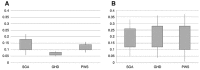
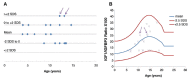
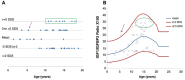
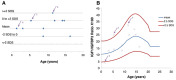
Results: Before rGH treatment, mean IGF1/IGFBP3 molar ratio in the SGA, GHD and PWS groups was 0.14±0.04, 0.07±0.01 and 0.12±0.02, respectively. After the initiation of rGH treatment, these averages were 0.19±0.07, 0.20±0.08 and 0.19±0.09, within the normal range for most children, even at puberty and despite some significant increases in serum IGF1 levels.
Conclusion: We consider IGF1/IGFBP3 molar ratio to be a useful additional parameter for assessing therapeutic safety in patients on rGH, and for maintaning the values within the normal range for age and pubertal stage.
Keywords: GH therapy; IGF1/IGFBP3 molar ratio; growth hormone deficiency; small for gestational age; Prader-Willi syndrome.
Figures
Similar articles
-
Elevated insulin-like growth factor-I values in children with Prader-Willi syndrome compared with growth hormone (GH) deficiency children over two years of GH treatment.J Clin Endocrinol Metab. 2010 Oct;95(10):4600-8. doi: 10.1210/jc.2009-1831. J Clin Endocrinol Metab. 2010. PMID: 20926543
-
Spontaneous growth hormone secretion and IGF1:IGFBP3 molar ratios in children born small for gestational age (SGA).Growth Horm IGF Res. 2004 Dec;14(6):455-61. doi: 10.1016/j.ghir.2004.08.002. Growth Horm IGF Res. 2004. PMID: 15519254
-
Functional and total IGFBP3 for the assessment of disorders of the GH/IGF1 axis in children with chronic kidney disease, GH deficiency, or short stature after SGA status at birth.Eur J Endocrinol. 2012 May;166(5):923-31. doi: 10.1530/EJE-11-0923. Epub 2012 Feb 8. Eur J Endocrinol. 2012. PMID: 22318747
-
Is there growth hormone deficiency in prader-willi Syndrome? Six arguments to support the presence of hypothalamic growth hormone deficiency in Prader-Willi syndrome.Horm Res. 2000;53 Suppl 3:44-52. doi: 10.1159/000023533. Horm Res. 2000. PMID: 10971104 Review.
-
Indications for growth hormone therapy in children.Arch Dis Child. 2012 Jan;97(1):63-8. doi: 10.1136/adc.2010.186205. Epub 2011 May 3. Arch Dis Child. 2012. PMID: 21540481 Review.
Cited by
-
Early psychomotor development and growth hormone therapy in children with Prader-Willi syndrome: a review.Eur J Pediatr. 2024 Mar;183(3):1021-1036. doi: 10.1007/s00431-023-05327-z. Epub 2023 Nov 21. Eur J Pediatr. 2024. PMID: 37987848 Review.
-
Declining Levels and Bioavailability of IGF-I in Cardiovascular Aging Associate With QT Prolongation-Results From the 1946 British Birth Cohort.Front Cardiovasc Med. 2022 Apr 22;9:863988. doi: 10.3389/fcvm.2022.863988. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35528832 Free PMC article.
-
Growth hormone treatment does not to lead to insulin resistance nor excessive rise in IGF-1 levels, while improving height in patients small for gestational age A long-term observational study.Clin Endocrinol (Oxf). 2022 Apr;96(4):558-568. doi: 10.1111/cen.14626. Epub 2021 Dec 9. Clin Endocrinol (Oxf). 2022. PMID: 34882803 Free PMC article.
-
Predictive value of IGF-1/IGFBP-3 ratio for thyroid nodules in type 2 diabetic mellitus.Front Endocrinol (Lausanne). 2024 Oct 9;15:1444279. doi: 10.3389/fendo.2024.1444279. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39444449 Free PMC article.
-
24-Week jumping exercise influence on growth speed and GH-IGF-1-IGFBP-3 axis among short-stature children.BMC Pediatr. 2025 Jul 1;25(1):476. doi: 10.1186/s12887-025-05821-3. BMC Pediatr. 2025. PMID: 40597964 Free PMC article.
References
-
- Sizonenko PC, Clayton PE, Cohen P, Hintz RL, Tanaka T, Laron Z. Diagnosis and management of growth hormone deficiency in childhood and adolescence. Part 1: diagnosis of growth hormone deficiency. Growth Horm IGF Res. 2001;11:137–165. - PubMed
-
- Hilczer M, Smyczynska J, Lewinski A. Monitoring and optimising of growth hormone (GH) therapy in GH-deficient children - the role of assessment of insulin-like growth factor-I (IGF-I) secretion and IGF-I/IGF binding protein-3 molar ratio. Med Sci Tech. 2006;47:219–223.
-
- Juul A, Bang P, Hertel NT, Main K, Dalgaard P, Jørgensen K, Müller J, Hall K, Skakkebaek NE. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin Endocrinol Metab. 1994;78:744–752. - PubMed
-
- Ranke MB, Traunecker R, Martin DD, Schweizer R, Schwarze CP, Wollmann HA, Binder G. IGF-I and IGF binding protein-3 levels during initial GH Dosage step-up are indicators of GH sensitivity in GH-deficient children and short children born small for gestational age. Horm Res. 2005;64:68–76. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
