Evaluation of IGF1/IGFBP3 Molar Ratio as an Effective Tool for Assessing the Safety of Growth Hormone Therapy in Small-for-gestational-age, Growth Hormone-Deficient and Prader-Willi Children
- PMID: 30759961
- PMCID: PMC6745465
- DOI: 10.4274/jcrpe.galenos.2019.2018.0277
Evaluation of IGF1/IGFBP3 Molar Ratio as an Effective Tool for Assessing the Safety of Growth Hormone Therapy in Small-for-gestational-age, Growth Hormone-Deficient and Prader-Willi Children
Abstract
Objective: IGF1 concentration is the most widely used parameter for the monitoring and therapeutic adaptation of recombinant human growth hormone (rGH) treatment. However, more than half the variation of the therapeutic response is accounted for by variability in the serum concentrations of IGF1 and IGFBP3. We therefore compared the use of IGF1/IGFBP3 molar ratio with that of IGF1 concentration alone.
Methods: We selected 92 children on rGH for this study and assigned them to three groups on the basis of growth deficiency etiology: small for gestational age (SGA), GH deficiency (GHD) and Prader-Willi syndrome (PWS). Plasma IGF1 and IGFBP3 concentrations and their molar ratio were determined.
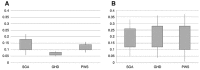
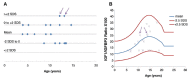
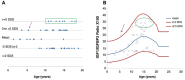
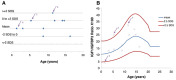
Results: Before rGH treatment, mean IGF1/IGFBP3 molar ratio in the SGA, GHD and PWS groups was 0.14±0.04, 0.07±0.01 and 0.12±0.02, respectively. After the initiation of rGH treatment, these averages were 0.19±0.07, 0.20±0.08 and 0.19±0.09, within the normal range for most children, even at puberty and despite some significant increases in serum IGF1 levels.
Conclusion: We consider IGF1/IGFBP3 molar ratio to be a useful additional parameter for assessing therapeutic safety in patients on rGH, and for maintaning the values within the normal range for age and pubertal stage.
Keywords: GH therapy; IGF1/IGFBP3 molar ratio; growth hormone deficiency; small for gestational age; Prader-Willi syndrome.
Figures
References
-
- Sizonenko PC, Clayton PE, Cohen P, Hintz RL, Tanaka T, Laron Z. Diagnosis and management of growth hormone deficiency in childhood and adolescence. Part 1: diagnosis of growth hormone deficiency. Growth Horm IGF Res. 2001;11:137–165. - PubMed
-
- Hilczer M, Smyczynska J, Lewinski A. Monitoring and optimising of growth hormone (GH) therapy in GH-deficient children - the role of assessment of insulin-like growth factor-I (IGF-I) secretion and IGF-I/IGF binding protein-3 molar ratio. Med Sci Tech. 2006;47:219–223.
-
- Juul A, Bang P, Hertel NT, Main K, Dalgaard P, Jørgensen K, Müller J, Hall K, Skakkebaek NE. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin Endocrinol Metab. 1994;78:744–752. - PubMed
-
- Ranke MB, Traunecker R, Martin DD, Schweizer R, Schwarze CP, Wollmann HA, Binder G. IGF-I and IGF binding protein-3 levels during initial GH Dosage step-up are indicators of GH sensitivity in GH-deficient children and short children born small for gestational age. Horm Res. 2005;64:68–76. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
