A paradigm shift in studies based on rheumatoid arthritis clinical registries
- PMID: 30759964
- PMCID: PMC6718765
- DOI: 10.3904/kjim.2018.440
A paradigm shift in studies based on rheumatoid arthritis clinical registries
Abstract
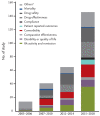
Clinical research is the study of aspects of patient health or illness that are closely related to clinical practice. In the late 20th and early 21th century, outcomes for patients with rheumatoid arthritis (RA) improved dramatically due to breakthroughs in new drugs. Patient-reported outcome measures now play a significant role in the drug development process as study endpoints in clinical trials of new therapies, and this has led to increased interest in the patient's perspective, drug safety and treatment outcomes in clinical practice. In accordance with these needs, many prospective cohorts for RA patients and registries of biologic disease modifying anti-rheumatic drugs have been actively conducted in the United States and European and Asian countries. A gradual shift is taking place in the major outcomes of clinical research using these prospective cohorts and registries. This article will introduce representative registries for RA in each country set up in the early 2000s and will discuss future perspectives in clinical research on RA patients using such clinical registries.
Keywords: Arthritis, rheumatoid; Big data; Cohort studies; Patients reported outcomes; Registries.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
Similar articles
-
Characteristics and outcomes of rheumatoid arthritis patients who started biosimilar infliximab.Rheumatol Int. 2017 Jun;37(6):1007-1014. doi: 10.1007/s00296-017-3663-z. Epub 2017 Feb 18. Rheumatol Int. 2017. PMID: 28214924
-
Comparative effectiveness of treatment options after conventional DMARDs failure in rheumatoid arthritis.Rheumatol Int. 2017 Jun;37(6):975-982. doi: 10.1007/s00296-016-3649-2. Epub 2017 Jan 28. Rheumatol Int. 2017. PMID: 28132102
-
The role of registries in the treatment of rheumatoid arthritis with biologic disease-modifying anti-rheumatic drugs.Pharmacol Res. 2019 Oct;148:104410. doi: 10.1016/j.phrs.2019.104410. Epub 2019 Aug 25. Pharmacol Res. 2019. PMID: 31461667 Review.
-
Head-to-head comparison of aggressive conventional therapy and three biological treatments and comparison of two de-escalation strategies in patients who respond to treatment: study protocol for a multicenter, randomized, open-label, blinded-assessor, phase 4 study.Trials. 2017 Apr 4;18(1):161. doi: 10.1186/s13063-017-1891-x. Trials. 2017. PMID: 28376912 Free PMC article. Clinical Trial.
-
Safety of anti-rheumatic drugs for rheumatoid arthritis in pregnancy and lactation.Int J Rheum Dis. 2016 Sep;19(9):834-43. doi: 10.1111/1756-185X.12860. Epub 2016 Apr 29. Int J Rheum Dis. 2016. PMID: 27125255 Review.
Cited by
-
From Rheumatology 1.0 to Rheumatology 4.0 and beyond: the contributions of Big Data to the field of rheumatology.Mediterr J Rheumatol. 2019 Mar;30(1):3-6. doi: 10.31138/mjr.30.1.3. Mediterr J Rheumatol. 2019. PMID: 31938766 Free PMC article. No abstract available.
References
-
- Furner SE, Hootman JM, Helmick CG, Bolen J, Zack MM. Health-related quality of life of US adults with arthritis: analysis of data from the behavioral risk factor surveillance system, 2003, 2005, and 2007. Arthritis Care Res (Hoboken) 2011;63:788–799. - PubMed
-
- Gladman DD, Farewell VT. Longitudinal cohort studies. J Rheumatol Suppl. 2005;72:30–32. - PubMed
-
- Willke RJ, Burke LB, Erickson P. Measuring treatment impact: a review of patient-reported outcomes and other efficacy endpoints in approved product labels. Control Clin Trials. 2004;25:535–552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
