Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update
- PMID: 30759967
- PMCID: PMC6759428
- DOI: 10.3350/cmh.2018.0090
Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update
Abstract
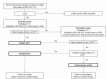
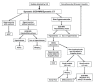
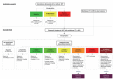
The goal of this review is to present the similarities and differences among the latest guidelines for noninvasive diagnosis of hepatocellular carcinoma (HCC) of American Association for the Study of Liver Disease (AASLD), European Association for the Study of the Liver (EASL), Liver Imaging Reporting and Data System (LI-RADS), Asian Pacific Association for the Study of the Liver (APASL), and Korean Liver Cancer Association- National Cancer Center (KLCA-NCC) of Korea. In 2018, major guideline updates have been proposed by the AASLD, EASL and KLCA-NCC; AASLD newly incorporated LI-RADS into their HCC diagnostic algorithm. The AASLD and EASL guidelines now include magnetic resonance imaging (MRI) using hepatobiliary contrast media as a first-line diagnostic test in addition to dynamic computed tomography and MRI using extracellular contrast media and the KLCA-NCC and EASL guidelines also include contrast-enhanced ultrasound as a second-line diagnostic test. We will comprehensively review the HCC surveillance and diagnostic algorithms and compare and highlight key features for each guideline. We also address limitations of current systems for the noninvasive diagnosis of HCC.
Keywords: Diagnosis; Guideline; Hepatocellular carcinoma; Standards.
Conflict of interest statement
Authors declare no conflicts of interest.
Figures


References
-
- Choo SP, Tan WL, Goh BKP, Tai WM, Zhu AX. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer. 2016;122:3430–3446. - PubMed
-
- Global Burden of Disease Liver Cancer Collaboration. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3:1683–1691. - PMC - PubMed
-
- Shields A, Reddy KR. Hepatocellular carcinoma: current treatment strategies. Curr Treat Options Gastroenterol. 2005;8:457–466. - PubMed
-
- Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. - PubMed
-
- Tang A, Cruite I, Mitchell DG, Sirlin CB. Hepatocellular carcinoma imaging systems: why they exist, how they have evolved, and how they differ. Abdom Radiol (NY) 2018;43:3–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

