Epigenetic Mechanisms in Monocytes/Macrophages Regulate Inflammation in Cardiometabolic and Vascular Disease
- PMID: 30760015
- PMCID: PMC6438376
- DOI: 10.1161/ATVBAHA.118.312135
Epigenetic Mechanisms in Monocytes/Macrophages Regulate Inflammation in Cardiometabolic and Vascular Disease
Abstract
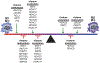
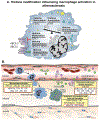
Cardiometabolic and vascular disease, with their associated secondary complications, are the leading cause of morbidity and mortality in Western society. Chronic inflammation is a common theme that underlies initiation and progression of cardiovascular disease. In this regard, monocytes/macrophages are key players in the development of a chronic inflammatory state. Over the past decade, epigenetic modifications, such as DNA methylation and posttranslational histone processing, have emerged as important regulators of immune cell phenotypes. Accumulating studies reveal the importance of epigenetic enzymes in the dynamic regulation of key signaling pathways that alter monocyte/macrophage phenotypes in response to environmental stimuli. In this review, we highlight the current paradigms of monocyte/macrophage polarization and the emerging role of epigenetic modification in the regulation of monocyte/macrophage phenotype in obesity, diabetes mellitus, atherosclerosis, and abdominal aortic aneurysms.
Keywords: atherosclerosis; inflammation; macrophages; monocytes; phenotype.
Conflict of interest statement
DISCLOSURES
The authors report no conflicts of interest
Figures

Similar articles
-
The Role of Epigenetic Modifications in Abdominal Aortic Aneurysm Pathogenesis.Biomolecules. 2022 Jan 21;12(2):172. doi: 10.3390/biom12020172. Biomolecules. 2022. PMID: 35204673 Free PMC article. Review.
-
Epigenetic regulation in monocyte/macrophage: A key player during atherosclerosis.Cardiovasc Ther. 2017 Jun;35(3). doi: 10.1111/1755-5922.12262. Cardiovasc Ther. 2017. PMID: 28371472 Review.
-
Epigenetic Regulation of S100A9 and S100A12 Expression in Monocyte-Macrophage System in Hyperglycemic Conditions.Front Immunol. 2020 Jun 2;11:1071. doi: 10.3389/fimmu.2020.01071. eCollection 2020. Front Immunol. 2020. PMID: 32582175 Free PMC article.
-
4-1BBL signaling promotes cell proliferation through reprogramming of glucose metabolism in monocytes/macrophages.FEBS J. 2015 Apr;282(8):1468-80. doi: 10.1111/febs.13236. Epub 2015 Mar 13. FEBS J. 2015. PMID: 25691217
-
Protein Thiol Redox Signaling in Monocytes and Macrophages.Antioxid Redox Signal. 2016 Nov 20;25(15):816-835. doi: 10.1089/ars.2016.6697. Epub 2016 Jul 13. Antioxid Redox Signal. 2016. PMID: 27288099 Free PMC article. Review.
Cited by
-
Deciphering the performance of macrophages in tumour microenvironment: a call for precision immunotherapy.J Hematol Oncol. 2024 Jun 11;17(1):44. doi: 10.1186/s13045-024-01559-0. J Hematol Oncol. 2024. PMID: 38863020 Free PMC article. Review.
-
Vascular homeostasis at high-altitude: role of genetic variants and transcription factors.Pulm Circ. 2020 Nov 19;10(4):2045894020913475. doi: 10.1177/2045894020913475. eCollection 2020 Oct-Dec. Pulm Circ. 2020. PMID: 33282179 Free PMC article. Review.
-
Regulation of histone H3K27 methylation in inflammation and cancer.Mol Biomed. 2025 Mar 5;6(1):14. doi: 10.1186/s43556-025-00254-x. Mol Biomed. 2025. PMID: 40042761 Free PMC article. Review.
-
Targeting Cell-Specific Molecular Mechanisms of Innate Immunity in Atherosclerosis.Front Physiol. 2022 Apr 1;13:802990. doi: 10.3389/fphys.2022.802990. eCollection 2022. Front Physiol. 2022. PMID: 35432000 Free PMC article. Review.
-
The Relationship between Body Mass Index, Obesity, and LINE-1 Methylation: A Cross-Sectional Study on Women from Southern Italy.Dis Markers. 2021 Dec 3;2021:9910878. doi: 10.1155/2021/9910878. eCollection 2021. Dis Markers. 2021. PMID: 34900031 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

