Echinococcosis: Advances in the 21st Century
- PMID: 30760475
- PMCID: PMC6431127
- DOI: 10.1128/CMR.00075-18
Echinococcosis: Advances in the 21st Century
Abstract
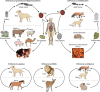
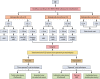
Echinococcosis is a zoonosis caused by cestodes of the genus Echinococcus (family Taeniidae). This serious and near-cosmopolitan disease continues to be a significant public health issue, with western China being the area of highest endemicity for both the cystic (CE) and alveolar (AE) forms of echinococcosis. Considerable advances have been made in the 21st century on the genetics, genomics, and molecular epidemiology of the causative parasites, on diagnostic tools, and on treatment techniques and control strategies, including the development and deployment of vaccines. In terms of surgery, new procedures have superseded traditional techniques, and total cystectomy in CE, ex vivo resection with autotransplantation in AE, and percutaneous and perendoscopic procedures in both diseases have improved treatment efficacy and the quality of life of patients. In this review, we summarize recent progress on the biology, epidemiology, diagnosis, management, control, and prevention of CE and AE. Currently there is no alternative drug to albendazole to treat echinococcosis, and new compounds are required urgently. Recently acquired genomic and proteomic information can provide a platform for improving diagnosis and for finding new drug and vaccine targets, with direct impact in the future on the control of echinococcosis, which continues to be a global challenge.
Keywords: Echinococcus; Echinococcus granulosus; Echinococcus multilocularis; alveolar echinococcosis; cystic echinococcosis; echinococcosis; genetic epidemiology; genome; strains/genotypes; transcriptome; zoonosis.
Copyright © 2019 American Society for Microbiology.
Figures








References
-
- Schweiger A, Ammann RW, Candinas D, Clavien PA, Eckert J, Gottstein B, Halkic N, Muellhaupt B, Prinz BM, Reichen J, Tarr PE, Torgerson PR, Deplazes P. 2007. Human alveolar echinococcosis after fox population increase, Switzerland. Emerg Infect Dis 13:878–882. doi: 10.3201/eid1306.061074. - DOI - PMC - PubMed
-
- Budke CM, Carabin H, Ndimubanzi PC, Nguyen H, Rainwater E, Dickey M, Bhattarai R, Zeziulin O, Qian MB. 2013. A systematic review of the literature on cystic echinococcosis frequency worldwide and its associated clinical manifestations. Am J Trop Med Hyg 88:1011–1027. doi: 10.4269/ajtmh.12-0692. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

