Kynurenine 3-monooxygenase is a critical regulator of renal ischemia-reperfusion injury
- PMID: 30760699
- PMCID: PMC6374422
- DOI: 10.1038/s12276-019-0210-x
Kynurenine 3-monooxygenase is a critical regulator of renal ischemia-reperfusion injury
Abstract
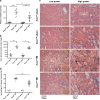
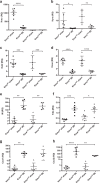
Acute kidney injury (AKI) following ischemia-reperfusion injury (IRI) has a high mortality and lacks specific therapies. Here, we report that mice lacking kynurenine 3-monooxygenase (KMO) activity (Kmonull mice) are protected against AKI after renal IRI. We show that KMO is highly expressed in the kidney and exerts major metabolic control over the biologically active kynurenine metabolites 3-hydroxykynurenine, kynurenic acid, and downstream metabolites. In experimental AKI induced by kidney IRI, Kmonull mice had preserved renal function, reduced renal tubular cell injury, and fewer infiltrating neutrophils compared with wild-type (Kmowt) control mice. Together, these data confirm that flux through KMO contributes to AKI after IRI, and supports the rationale for KMO inhibition as a therapeutic strategy to protect against AKI during critical illness.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

