Highly Efficient Transgenesis in Ferrets Using CRISPR/Cas9-Mediated Homology-Independent Insertion at the ROSA26 Locus
- PMID: 30760763
- PMCID: PMC6374392
- DOI: 10.1038/s41598-018-37192-4
Highly Efficient Transgenesis in Ferrets Using CRISPR/Cas9-Mediated Homology-Independent Insertion at the ROSA26 Locus
Abstract
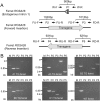
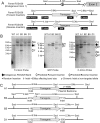
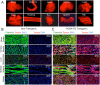
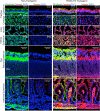
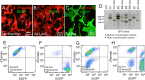
The domestic ferret (Mustela putorius furo) has proven to be a useful species for modeling human genetic and infectious diseases of the lung and brain. However, biomedical research in ferrets has been hindered by the lack of rapid and cost-effective methods for genome engineering. Here, we utilized CRISPR/Cas9-mediated, homology-independent insertion at the ROSA26 "safe harbor" locus in ferret zygotes and created transgenic animals expressing a dual-fluorescent Cre-reporter system flanked by PhiC31 and Bxb1 integrase attP sites. Out of 151 zygotes injected with circular transgene-containing plasmid and Cas9 protein loaded with the ROSA26 intron-1 sgRNA, there were 23 births of which 5 had targeted integration events (22% efficiency). The encoded tdTomato transgene was highly expressed in all tissues evaluated. Targeted integration was verified by PCR analyses, Southern blot, and germ-line transmission. Function of the ROSA26-CAG-LoxPtdTomatoStopLoxPEGFP (ROSA-TG) Cre-reporter was confirmed in primary cells following Cre expression. The Phi31 and Bxb1 integrase attP sites flanking the transgene will also enable rapid directional insertion of any transgene without a size limitation at the ROSA26 locus. These methods and the model generated will greatly enhance biomedical research involving lineage tracing, the evaluation of stem cell therapy, and transgenesis in ferret models of human disease.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

