Antimicrobial and inflammatory properties of South African clinical Lactobacillus isolates and vaginal probiotics
- PMID: 30760770
- PMCID: PMC6374385
- DOI: 10.1038/s41598-018-38253-4
Antimicrobial and inflammatory properties of South African clinical Lactobacillus isolates and vaginal probiotics
Abstract
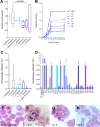
Bacterial vaginosis (BV) causes genital inflammation and increased HIV acquisition risk. The standard-of-care for BV, antibiotic therapy, is associated with high recurrence rates. Probiotics may improve treatment outcomes, although substantial heterogeneity in efficacy has been observed during clinical trials. To evaluate the potential to improve existing probiotics, we compared the inflammatory and antimicrobial (adhesion, H2O2, D-lactate and L-lactate production) characteristics of 23 vaginal Lactobacillus isolates from South African women, commercial vaginal probiotics (L. casei rhamnosus, L. acidophilus) and 4 reference strains. All lactobacilli induced inflammatory cytokine production by genital epithelial cells and produced D-lactate. Of six isolates assessed, five suppressed inflammatory responses to Gardnerella vaginalis. Although the L. acidophilus probiotic was the most adherent, many clinical isolates produced greater amounts of H2O2, D-lactate and L-lactate than the probiotics. The most L-lactate and H2O2 were produced by L. jensenii (adjusted p = 0.0091) and L. mucosae (adjusted p = 0.0308) species, respectively. According to the characteristics evaluated, the top 10 isolates included 4 L. jensenii, 2 L. crispatus, 1 L. mucosae, 1 L. vaginalis and the L. acidophilus probiotic. There is potential to develop an improved vaginal probiotic using clinical Lactobacillus isolates. Inflammatory profiles are critical to evaluate as some isolates induced substantial cytokine production.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

