Tumorigenicity assay essential for facilitating safety studies of hiPSC-derived cardiomyocytes for clinical application
- PMID: 30760836
- PMCID: PMC6374479
- DOI: 10.1038/s41598-018-38325-5
Tumorigenicity assay essential for facilitating safety studies of hiPSC-derived cardiomyocytes for clinical application
Abstract
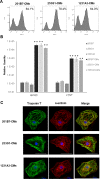
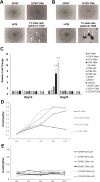
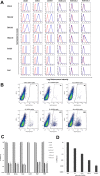
Transplantation of cardiomyocytes (CMs) derived from human induced pluripotent stem cells (hiPSC-CMs) is a promising treatment for heart failure, but residual undifferentiated hiPSCs and malignant transformed cells may lead to tumor formation. Here we describe a highly sensitive tumorigenicity assay for the detection of these cells in hiPSC-CMs. The soft agar colony formation assay and cell growth analysis were unable to detect malignantly transformed cells in hiPSC-CMs. There were no karyotypic abnormalities during hiPSCs subculture and differentiation. The hiPSC markers TRA1-60 and LIN28 showed the highest sensitivity for detecting undifferentiated hiPSCs among primary cardiomyocytes. Transplantation of hiPSC-CMs with a LIN28-positive fraction > 0.33% resulted in tumor formation in nude rats, whereas no tumors were formed when the fraction was < 0.1%. These findings suggested that combination of these in vitro and in vivo tumorigenecity assays can verify the safety of hiPSC-CMs for cell transplantation therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Miyagawa S. Translational research in iPS cell derived cardiomyocyte sheet for the treatment of heart failure. Nihon Rinsho. 2016;74:667–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

