Applications of Light-Sheet Microscopy in Microdevices
- PMID: 30760983
- PMCID: PMC6362405
- DOI: 10.3389/fnana.2019.00001
Applications of Light-Sheet Microscopy in Microdevices
Abstract
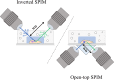
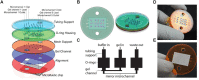
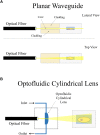
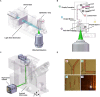
Light-sheet fluorescence microscopy (LSFM) has been present in cell biology laboratories for quite some time, mainly as custom-made systems, with imaging applications ranging from single cells (in the micrometer scale) to small organisms (in the millimeter scale). Such microscopes distinguish themselves for having very low phototoxicity levels and high spatial and temporal resolution, properties that make them ideal for a large range of applications. These include the study of cellular dynamics, in particular cellular motion which is essential to processes such as tumor metastasis and tissue development. Experimental setups make extensive use of microdevices (bioMEMS) that provide better control over the substrate environment than traditional cell culture experiments. For example, to mimic in vivo conditions, experiment biochemical dynamics, and trap, move or count cells. Microdevices provide a higher degree of empirical complexity but, so far, most have been designed to be imaged through wide-field or confocal microscopes. Nonetheless, the properties of LSFM render it ideal for 3D characterization of active cells. When working with microdevices, confocal microscopy is more widespread than LSFM even though it suffers from higher phototoxicity and slower acquisition speeds. It is sometimes possible to illuminate with a light-sheet microdevices designed for confocal microscopes. However, these bioMEMS must be redesigned to exploit the full potential of LSFM and image more frequently on a wider scale phenomena such as motion, traction, differentiation, and diffusion of molecules. The use of microdevices for LSFM has extended beyond cell tracking studies into experiments regarding cytometry, spheroid cultures and lab-on-a-chip automation. Due to light-sheet microscopy being in its early stages, a setup of these characteristics demands some degree of optical expertise; and designing three-dimensional microdevices requires facilities, ingenuity, and experience in microfabrication. In this paper, we explore different approaches where light-sheet microscopy can achieve single-cell and subcellular resolution within microdevices, and provide a few pointers on how these experiments may be improved.
Keywords: SPIM; cellular imaging; light-sheet fluorescence microscopy; microdevices; microfluidics.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

