Bestrophin-3 Expression in a Subpopulation of Astrocytes in the Neonatal Brain After Hypoxic-Ischemic Injury
- PMID: 30761013
- PMCID: PMC6362097
- DOI: 10.3389/fphys.2019.00023
Bestrophin-3 Expression in a Subpopulation of Astrocytes in the Neonatal Brain After Hypoxic-Ischemic Injury
Abstract
Bestrophin-3, a potential candidate for a calcium-activated chloride channel, recently was suggested to have cell-protective functions. We studied the expression and alternative splicing of bestrophin-3 in neonatal mouse brain and after hypoxic-ischemic (HI) injury and in human neonatal brain samples. HI brain injury was induced in 9-day old mice by unilateral permanent common carotid artery occlusion in combination with exposure to 10% oxygen for 50 min. Endoplasmic reticulum stress was induced by thapsigargin treatment in primary culture of mouse brain astrocytes. We also investigated expression of bestrophin-3 protein in a sample of human neonatal brain tissue. Bestrophin-3 protein expression was detected with immunohistochemical methods and western blot; mRNA expression and splicing were analyzed by RT-PCR. HI induced a brain tissue infarct and a pronounced increase in the endoplasmic reticulum-associated marker CHOP. Three days after HI a population of astrocytes co-expressed bestrophin-3 and nestin in a penumbra-like area of the injured hemisphere. However, total levels of Bestrophin-3 protein in mouse cortex were reduced after injury. Mouse astrocytes in primary culture also expressed bestrophin-3 protein, the amount of which was reduced by endoplasmic reticulum stress. Bestrophin-3 protein was detected in astrocytes in the hippocampal region of the human neonatal brain which had patchy white matter gliosis and neuronal loss in the Sommer's sector of the Ammon's horn (CA1). Analysis of bestrophin-3 mRNA in mouse brain with and without injury showed the presence of two truncated spliced variants, but no full-length mRNA. Total amount of bestrophin-3 mRNA increased after HI, but showed only minor injury-related change. However, the splice variants of bestrophin-3 mRNA were differentially regulated after HI depending on the presence of tissue injury. Our results show that bestrophin-3 is expressed in neonatal mouse brain after injury and in the human neonatal brain with pathology. In mouse brain bestrophin-3 protein is upregulated in a specific astrocyte population after injury and is co-expressed with nestin. Splice variants of bestrophin-3 mRNA respond differently to HI, which might indicate their different roles in tissue injury.
Keywords: alternative splicing; astroglia; bestrophin-3 (Best3); endoplasmic reticulum (ER) stress; hypoxia-ischemia.
Figures

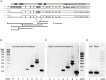

 ), between injured and sham (L vs. sham group at zero,
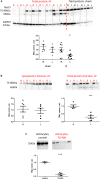
), between injured and sham (L vs. sham group at zero,  ), and between uninjured and injured (R vs. L, ×), all symbols indicate P < 0.05. The total mRNA for Best3 increased in both hemispheres at early time points after HI, but returned to control values already 24 h after HI, and even showed a transient decrease at 72 h (A). The long “+6” splice variant increased only in the uninjured hemisphere, while in the injured hemisphere it decreased and stayed decreased through all time point except 24 h (B). The short “–6” splice variant transiently increased at 6 and 12 h, and in the injured hemisphere also again at 72 h (C). Expression of mRNA for nestin (D) decreased at early time points in non-injured hemisphere, but returned to control values at 72 h after HI, while it increased in the injured hemisphere during this period to return to control values at 7 days after injury. The ER-stress marker CHOP slightly decreased during the early period after HI in the non-injured hemisphere, but was elevated throughout the studied period in the injured hemisphere (E). A total of 41 pups (26 HI, 15 sham) were used; for details see section “Materials and Methods.” Detailed statistical results are given in the (Supplementary Table S1).
), and between uninjured and injured (R vs. L, ×), all symbols indicate P < 0.05. The total mRNA for Best3 increased in both hemispheres at early time points after HI, but returned to control values already 24 h after HI, and even showed a transient decrease at 72 h (A). The long “+6” splice variant increased only in the uninjured hemisphere, while in the injured hemisphere it decreased and stayed decreased through all time point except 24 h (B). The short “–6” splice variant transiently increased at 6 and 12 h, and in the injured hemisphere also again at 72 h (C). Expression of mRNA for nestin (D) decreased at early time points in non-injured hemisphere, but returned to control values at 72 h after HI, while it increased in the injured hemisphere during this period to return to control values at 7 days after injury. The ER-stress marker CHOP slightly decreased during the early period after HI in the non-injured hemisphere, but was elevated throughout the studied period in the injured hemisphere (E). A total of 41 pups (26 HI, 15 sham) were used; for details see section “Materials and Methods.” Detailed statistical results are given in the (Supplementary Table S1).
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

