Hormonal Regulation of Follicle-Stimulating Hormone Glycosylation in Males
- PMID: 30761084
- PMCID: PMC6361742
- DOI: 10.3389/fendo.2019.00017
Hormonal Regulation of Follicle-Stimulating Hormone Glycosylation in Males
Abstract
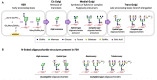
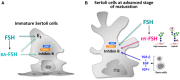
The Follicle-Stimulating Hormone plays an important role in the regulation of gametogenesis. It is synthesized and secreted as a family of glycoforms with differing oligosaccharide structure, biological action, and half-life. The presence of these oligosaccharides is absolutely necessary for the full expression of hormone bioactivity at the level of the target cell. The endocrine milieu modulates the glycosylation of this hormone. During male sexual development a progressive increase in FSH sialylation and in the proportion of glycoforms bearing complex oligosaccharides are the main features in this physiological condition. In late puberty, FSH oligosaccharides are largely processed in the medial- and trans-Golgi cisternae of the gonadotrope and remain without changes throughout adult life. In experimental models, the absence of gonads severely affects FSH sialylation; androgen administration is able to restore the characteristics observed under physiological conditions. The expression of ST6 beta-galactoside alpha-2,6-sialyltransferase 1 is hormonally regulated in the male rat; it decreases after short periods of castration but increases markedly at longer periods of androgen deprivation. Although ST3 beta-galactoside alpha-2,3-sialyltransferase 3 is expressed in the male rat pituitary it is not influenced by changes in the endocrine milieu. The oligosaccharide structure of FSH has an impact on the Sertoli cell endocrine activity. In more advanced stages of Sertoli cell maturation, both sialylation and complexity of the oligosaccharides are involved in the regulation of inhibin B production; moreover, FSH glycoforms bearing incomplete oligosaccharides may enhance the stimulatory effect exerted by gonadal growth factors. In this review, we discuss available information on variation of FSH glycosylation and its hormonal regulation under different physiological and experimental conditions, as well as the effect on Sertoli cell endocrine activity.
Keywords: Follicle-stimulating hormone glycosylation; Sertoli cell; hormonal regulation; inhibin; male gonad.
Figures
References
-
- Dahl KD, Stone MP. FSH isoforms, radioimmunoassays, bioassays, and their significance. J Androl. (1992) 13:11–22. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

