Sugar Sensing and Signaling in Candida albicans and Candida glabrata
- PMID: 30761119
- PMCID: PMC6363656
- DOI: 10.3389/fmicb.2019.00099
Sugar Sensing and Signaling in Candida albicans and Candida glabrata
Abstract
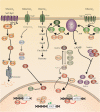
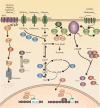
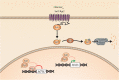
Candida species, such as Candida albicans and Candida glabrata, cause infections at different host sites because they adapt their metabolism depending on the available nutrients. They are able to proliferate under both nutrient-rich and nutrient-poor conditions. This adaptation is what makes these fungi successful pathogens. For both species, sugars are very important nutrients and as the sugar level differs depending on the host niche, different sugar sensing systems must be present. Saccharomyces cerevisiae has been used as a model for the identification of these sugar sensing systems. One of the main carbon sources for yeast is glucose, for which three different pathways have been described. First, two transporter-like proteins, ScSnf3 and ScRgt2, sense glucose levels resulting in the induction of different hexose transporter genes. This situation is comparable in C. albicans and C. glabrata, where sensing of glucose by CaHgt4 and CgSnf3, respectively, also results in hexose transporter gene induction. The second glucose sensing mechanism in S. cerevisiae is via the G-protein coupled receptor ScGpr1, which causes the activation of the cAMP/PKA pathway, resulting in rapid adaptation to the presence of glucose. The main components of this glucose sensing system are also conserved in C. albicans and C. glabrata. However, it seems that the ligand(s) for CaGpr1 are not sugars but lactate and methionine. In C. glabrata, this pathway has not yet been investigated. Finally, the glucose repression pathway ensures repression of respiration and repression of the use of alternative carbon sources. This pathway is not well characterized in Candida species. It is important to note that, apart from glucose, other sugars and sugar-analogs, such as N-acetylglucosamine in the case of C. albicans, are also important carbon sources. In these fungal pathogens, sensing sugars is important for a number of virulence attributes, including adhesion, oxidative stress resistance, biofilm formation, morphogenesis, invasion, and antifungal drug tolerance. In this review, the sugar sensing and signaling mechanisms in these Candida species are compared to S. cerevisiae.
Keywords: Candida albicans; Candida glabrata; Gpr1/Gpa2; Saccharomyces cerevisiae; Snf1/Mig1; Snf3/Rgt2-Hgt4; sugar sensing; sugar transport.
Figures

References
-
- Arendrup M. C. (2013). Candida and candidaemia. Susceptibility and epidemiology. Dan. Med. J. 60:B4698. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

