A polyextremophilic alcohol dehydrogenase from the Atlantis II Deep Red Sea brine pool
- PMID: 30761247
- PMCID: PMC6356862
- DOI: 10.1002/2211-5463.12557
A polyextremophilic alcohol dehydrogenase from the Atlantis II Deep Red Sea brine pool
Abstract
Enzymes originating from hostile environments offer exceptional stability under industrial conditions and are therefore highly in demand. Using single-cell genome data, we identified the alcohol dehydrogenase (ADH) gene, adh/a1a, from the Atlantis II Deep Red Sea brine pool. ADH/A1a is highly active at elevated temperatures and high salt concentrations (optima at 70 °C and 4 m KCl) and withstands organic solvents. The polyextremophilic ADH/A1a exhibits a broad substrate scope including aliphatic and aromatic alcohols and is able to reduce cinnamyl-methyl-ketone and raspberry ketone in the reverse reaction, making it a possible candidate for the production of chiral compounds. Here, we report the affiliation of ADH/A1a to a rare enzyme family of microbial cinnamyl alcohol dehydrogenases and explain unique structural features for halo- and thermoadaptation.
Keywords: alcohol dehydrogenase; extremophiles; extremozyme; halophiles; thermophiles.
Figures


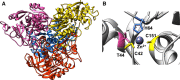

References
-
- Matsuda T, Yamanaka R and Nakamura K (2009) Recent progress in biocatalysis for asymmetric oxidation and reduction. Tetrahedron: Asymmetry 20, 513–557.
-
- Huisman GW, Liang J and Krebber A (2010) Practical chiral alcohol manufacture using ketoreductases. Curr Opin Chem Biol 14, 122–129. - PubMed
-
- Zhang R, Xu Y and Xiao R (2015) Redesigning alcohol dehydrogenases/reductases for more efficient biosynthesis of enantiopure isomers. Biotechnol Adv 33, 1671–1684. - PubMed
-
- Karan R and Khare SK (2010) Purification and characterization of a solvent‐stable protease from Geomicrobium sp. EMB2. Environ Technol 31, 1061–1072. - PubMed
-
- Eijsink VGH, Gåseidnes S, Borchert TV and van den Burg B (2005) Directed evolution of enzyme stability. Biomol Eng 22, 21–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

