Discovery of Pathologic GPCR Aggregation
- PMID: 30761305
- PMCID: PMC6363654
- DOI: 10.3389/fmed.2019.00009
Discovery of Pathologic GPCR Aggregation
Abstract
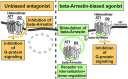
The family of G-protein-coupled receptors (GPCRs) is one of the most important drug targets. Mechanisms underlying GPCR activation and signaling are therefore of great pharmacologic interest. It was long thought that GPCRs exist and function as monomers. This feature was considered to distinguish GPCRs from other membrane receptors such as receptor tyrosine kinases or cytokine receptors, which signal from dimeric receptor complexes. But during the last two decades it was increasingly recognized that GPCRs can undergo aggregation to form dimers and higher order oligomers, resulting in homomeric and/or heteromeric protein complexes with different stoichiometries. Moreover, this protein complex formation could modify GPCR signaling and function. We contributed to this paradigm shift in GPCR pharmacology by the discovery of the first pathologic GPCR aggregation, which is the protein complex formation between the angiotensin II AT1 receptor and the bradykinin B2 receptor. Increased AT1-B2 heteromerization accounts for the angiotensin II hypersensitivity of pregnant women with preeclampsia hypertension. Since the discovery of AT1-B2, other pathologic GPCR aggregates were found, which contribute to atherosclerosis, neurodegeneration and Alzheimer's disease. As a result of our findings, pathologic GPCR aggregation appears as an independent and disease-specific process, which is increasingly considered as a novel target for pharmacologic intervention.
Keywords: Alzheimer's disease; G-protein-coupled receptor; atherosclerosis; beta-arrestin; biased agonist; neurodegeneration; oligomerization; preeclampsia.
Figures
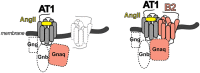
References
-
- Dixon RA, Kobilka BK, Strader DJ, Benovic JL, Dohlman HG, Frielle T, et al. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature (1986) 321:75–9. - PubMed
-
- Schertler GF, Villa C, Henderson R. Projection structure of rhodopsin. Nature (1983) 362:770–72. - PubMed
-
- Heldin CH. Dimerization of cell surface receptors in signal transduction. Cell (1995) 80:213–23. - PubMed
-
- Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. (1987) 56:615–49. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

