Caffeine and oocyte vitrification: Sheep as an animal model
- PMID: 30761320
- PMCID: PMC6161861
- DOI: 10.1016/j.ijvsm.2018.01.004
Caffeine and oocyte vitrification: Sheep as an animal model
Abstract
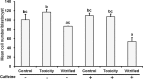
Oocyte cryopreservation is valuable way of preserving the female germ line. Vitrification of immature ovine oocytes decreased the levels of both maturation promoting factor (MPF) and mitogen-activated protein kinase (MAPK) in metaphase II (MII) oocytes after IVM. Our aims were 1) to evaluate the effects of vitrification of ovine GV-oocytes on spindle assembly, MPF/MAP kinases activities, and preimplantation development following IVM and IVF, 2) to elucidate the impact of caffeine supplementation during IVM on the quality and development of vitrified/warmed ovine GV-oocytes. Cumulus-oocyte complexes (COCs) from mature ewes were divided into vitrified, toxicity and control groups. Oocytes from each group were matured in vitro for 18 h in caffeine free IVM medium and denuded oocytes were incubated in maturation medium supplemented with 10 mM (+) or without (-) caffeine for another 6 h. At 24 h.p.m., oocytes were evaluated for spindle configuration, MPF/MAP kinases activities or fertilized and cultured in vitro for 7 days. Caffeine supplementation did not significantly affect the percentages of oocytes with normal spindle assembly in all the groups. Caffeine supplementation during IVM did not increase the activities of both kinases in vitrified groups. Cleavage and blastocyst development were significantly lower in vitrified groups than in control. Caffeine supplementation during the last 6 h of IVM did not significantly improve the cleavage and blastocyst rates in vitrified group. In conclusion, caffeine treatment during in vitro maturation has no positive impact on the quality and development of vitrified/warmed ovine GV-oocytes after IVM/IVF and embryo culture.
Keywords: Caffeine; GV; MPF/MAPK; Oocytes; Ovine; Vitrification.
Figures


Similar articles
-
Improved cryotolerance and developmental potential of in vitro and in vivo matured mouse oocytes by supplementing with a glutathione donor prior to vitrification.Mol Hum Reprod. 2016 Dec;22(12):867-881. doi: 10.1093/molehr/gaw059. Epub 2016 Sep 7. Mol Hum Reprod. 2016. PMID: 27604460
-
Effect of Disaccharide Inclusion in Vitrification and Warming Solutions on Developmental Competence of Vitrified/Warmed Germinal Vesicle Stage Buffalo Oocytes.Cryo Letters. 2020 Nov-Dec;41(6):351-357. Cryo Letters. 2020. PMID: 33990812
-
l-carnitine supplementation during vitrification of mouse germinal vesicle stage-oocytes and their subsequent in vitro maturation improves meiotic spindle configuration and mitochondrial distribution in metaphase II oocytes.Hum Reprod. 2014 Oct 10;29(10):2256-68. doi: 10.1093/humrep/deu201. Epub 2014 Aug 11. Hum Reprod. 2014. PMID: 25113843
-
Chapter 3 Current Challenges in Immature Oocyte Cryopreservation.Methods Mol Biol. 2017;1568:33-44. doi: 10.1007/978-1-4939-6828-2_3. Methods Mol Biol. 2017. PMID: 28421487 Review.
-
Microenvironment factors promoting the quality of vitrified cat oocytes.Theriogenology. 2023 Jan 15;196:275-283. doi: 10.1016/j.theriogenology.2022.11.027. Epub 2022 Nov 21. Theriogenology. 2023. PMID: 36442286 Review.
Cited by
-
Oocyte Cryopreservation in Domestic Animals and Humans: Principles, Techniques and Updated Outcomes.Animals (Basel). 2021 Oct 13;11(10):2949. doi: 10.3390/ani11102949. Animals (Basel). 2021. PMID: 34679970 Free PMC article. Review.
-
New Strategies for Conservation of Gentile di Puglia Sheep Breed, an Autochthonous Capital of Millennial Tradition in Southern Italy.Animals (Basel). 2023 Jul 20;13(14):2371. doi: 10.3390/ani13142371. Animals (Basel). 2023. PMID: 37508148 Free PMC article.
-
Effects of Cryoprotectant Concentration and Exposure Time during Vitrification of Immature Pre-Pubertal Lamb Cumulus-Oocyte Complexes on Nuclear and Cytoplasmic Maturation.Animals (Basel). 2024 Aug 14;14(16):2351. doi: 10.3390/ani14162351. Animals (Basel). 2024. PMID: 39199884 Free PMC article.
References
-
- Kopeika J., Thornhill A., Khalaf Y. The effect of cryopreservation on the genome of gametes and embryos: principles of cryobiology and critical appraisal of the evidence. Hum Reprod Update. 2015;21(2):209–227. PubMed PMID: 25519143. - PubMed
-
- Loutradi K.E., Kolibianakis E.M., Venetis C.A., Papanikolaou E.G., Pados G., Bontis I. Cryopreservation of human embryos by vitrification or slow freezing: a systematic review and meta-analysis. Fertil Steril. 2008;90(1):186–193. PubMed PMID: 17980870. - PubMed
-
- Chian R.C., Gilbert L., Huang J.Y., Demirtas E., Holzer H., Benjamin A. Live birth after vitrification of in vitro matured human oocytes. Fertil Steril. 2009;91(2):372–376. PubMed PMID: 18514195. - PubMed
-
- Nagy Z.P., Chang C.C., Shapiro D.B., Bernal D.P., Kort H.I., Vajta G. The efficacy and safety of human oocyte vitrification. Semin Reprod Med. 2009;27(6):450–455. PubMed PMID: 19806513. - PubMed
-
- Noyes N., Knopman J., Labella P., McCaffrey C., Clark-Williams M., Grifo J. Oocyte cryopreservation outcomes including pre-cryopreservation and post-thaw meiotic spindle evaluation following slow cooling and vitrification of human oocytes. Fertil Steril. 2010;94(6):2078–2082. PubMed PMID: 20188356. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

