Detection of Sarcocystis aucheniae in blood of llama using a duplex semi-nested PCR assay and its association with cyst infestation
- PMID: 30761363
- PMCID: PMC6286652
- DOI: 10.1016/j.heliyon.2018.e00928
Detection of Sarcocystis aucheniae in blood of llama using a duplex semi-nested PCR assay and its association with cyst infestation
Abstract
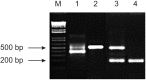
The protozoon Sarcocystis aucheniae is the causative agent of South American camelid (SAC) sarcocystosis. Infections are characterized by the presence of cysts in muscles which are in size and appearance similar to rice grains. As consumption of insufficiently cooked infected meat produces gastroenteritis, cyst-containing SAC meat is confiscated by sanitary authorities or depreciated with serious economic consequences for SAC breeders. In this work, a duplex semi-nested PCR was designed to simultaneously detect parasite and llama DNA in host blood samples. Species-specific regions of S. aucheniae 18S rRNA gene and Lama glama 16S mitochondrial gene were amplified, yielding bands of 583 and 257 bp, respectively, and separated by gel electrophoresis. The method proved to be highly sensitive, with a detection limit lower than one parasite per milliliter blood, and the inclusion of primers to detect llama-specific DNA resulted useful as a methodological control. Blood samples collected from llamas of Argentina and Bolivia (n = 225) were analyzed using this method, and 18.7 % resulted positive for S. aucheniae. No correlation was found between PCR results and llama age, sex or the finding of macroscopic cysts in meat after slaughter. Lack of molecular detection in the blood of some llamas harboring macrocysts suggests that parasite circulation in the bloodstream after encystment is under the detection threshold of the test or even absent, while PCR positive results in cyst-infested animals suggests that prior exposure to the parasite does not impede subsequent infections. The described method can be useful to detect active foci of infection, to assess the effectiveness of parasiticide treatments, and for the surveillance and tracing of definitive hosts.
Keywords: Bioinformatics; Microbiology; Molecular biology.
Figures

Similar articles
-
Molecular detection of Sarcocystis aucheniae in the blood of llamas from Argentina.Rev Argent Microbiol. 2016 Jul-Sep;48(3):200-205. doi: 10.1016/j.ram.2016.03.009. Epub 2016 Sep 8. Rev Argent Microbiol. 2016. PMID: 27615713
-
Molecular identification of Sarcocystis aucheniae as the macrocyst-forming parasite of llamas.Vet Parasitol. 2013 Dec 6;198(3-4):396-400. doi: 10.1016/j.vetpar.2013.09.007. Epub 2013 Sep 19. Vet Parasitol. 2013. PMID: 24129069
-
Sarcocystis masoni, n. sp. (Apicomplexa: Sarcocystidae), and redescription of Sarcocystis aucheniae from llama (Lama glama), guanaco (Lama guanicoe) and alpaca (Vicugna pacos).Parasitology. 2016 Apr;143(5):617-26. doi: 10.1017/S003118201600007X. Epub 2016 Mar 2. Parasitology. 2016. PMID: 26932444
-
Bovine sarcocystosis: Sarcocystis species, diagnosis, prevalence, economic and public health considerations, and association of Sarcocystis species with eosinophilic myositis in cattle.Int J Parasitol. 2023 Aug;53(9):463-475. doi: 10.1016/j.ijpara.2022.09.009. Epub 2022 Dec 1. Int J Parasitol. 2023. PMID: 36462560 Review.
-
Human infections with Sarcocystis species.Clin Microbiol Rev. 2015 Apr;28(2):295-311. doi: 10.1128/CMR.00113-14. Clin Microbiol Rev. 2015. PMID: 25715644 Free PMC article. Review.
Cited by
-
Detection of Three Sarcocystis Species (Apicomplexa) in Blood Samples of the Bank Vole and Yellow-Necked Mouse from Lithuania.Life (Basel). 2024 Mar 10;14(3):365. doi: 10.3390/life14030365. Life (Basel). 2024. PMID: 38541690 Free PMC article.
-
Sarcocystis spp. of New and Old World Camelids: Ancient Origin, Present Challenges.Pathogens. 2024 Feb 23;13(3):196. doi: 10.3390/pathogens13030196. Pathogens. 2024. PMID: 38535539 Free PMC article. Review.
-
Molecular identification of Sarcocystis aucheniae in the wild South American camelid Vicugna vicugna.Vet Res Commun. 2024 Oct;48(5):3429-3435. doi: 10.1007/s11259-024-10491-0. Epub 2024 Aug 9. Vet Res Commun. 2024. PMID: 39120675
-
Detection of Sarcocystis halieti DNA in the Blood of Western House Martin (Delichon urbicum) and Barn Swallow (Hirundo rustica) from Lithuania, and in Eurasian Griffon Vulture (Gyps fulvus) from Greece.Acta Parasitol. 2025 Aug 13;70(4):180. doi: 10.1007/s11686-025-01119-7. Acta Parasitol. 2025. PMID: 40801983
References
-
- Carletti T., Martin M., Romero S., Morrison D.A., Marcoppido G., Florin-Christensen M., Schnittger L. Molecular identification of Sarcocystis aucheniae as the macrocyst-forming parasite of llamas. Vet. Parasitol. 2013;198:396–400. - PubMed
-
- Castro C.E., Sam R., López U., González A., Silva M. Evaluación de la edad como factor de riesgo de la seropositividad a Sarcocystis sp. en alpacas. [Evaluation of age as a risk factor for seropositivity in Sarcocystis sp. in alpacas] Rev Inv Vet Perú. 2004;15(1):83–86.
-
- Choque J., Chávez V., Pachecho A., Leyva V., Panez J., Ticona D. Frequency of Sarcocystis sp. in sheepdogs from alpaca association breeders, Maranganí, Cusco. Rev. Inv. Vet. Perú. 2007;18(1):84–88.
-
- Cornejo R., Chávez A., Leyva V., Falcón N., Panez S., Ticona D. Relación entre el tamaño de los macroquistes de Sarcocystis aucheniae y su viabilidad en Canis familiaris. Relationship between the size of the macrocysts of Sarcocystis aucheniae and its viability in Canis familiarisRev. Investig. Vet. Perú. 2007;18(1):76–83.
-
- Decker Franco C., Florin-Christensen M., Schnittger L. Sarcocystis. In: Florin-Christensen M., Schnittger L., editors. Parasitic Protozoa of Farm Animals and Pets. Springer International Publishing AG; 2018. pp. 103–124.
LinkOut - more resources
Full Text Sources
Research Materials

