HCMV latency: what regulates the regulators?
- PMID: 30761409
- PMCID: PMC6647427
- DOI: 10.1007/s00430-019-00581-1
HCMV latency: what regulates the regulators?
Abstract
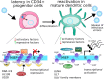
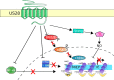
Human cytomegalovirus (HCMV) latency and reactivation is regulated by the chromatin structure at the major immediate early promoter (MIEP) within myeloid cells. Both cellular and viral factors are known to control this promoter during latency, here we will review the known mechanisms for MIEP regulation during latency. We will then focus on the virally encoded G-protein coupled receptor, US28, which suppresses the MIEP in early myeloid lineage cells. The importance of this function is underlined by the fact that US28 is essential for HCMV latency in CD34+ progenitor cells and CD14+ monocytes. We will describe cellular signalling pathways modulated by US28 to direct MIEP suppression during latency and demonstrate how US28 is able to 'regulate the regulators' of HCMV latency. Finally, we will describe how cell-surface US28 can be a target for antiviral therapies directed at the latent viral reservoir.
Keywords: Cell signalling; Chromatin; Cytomegalovirus; Latency; US28; Viral reservoir.
Conflict of interest statement
The authors declare they have no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

