Activation of Protein Kinase A Stimulates SUMOylation, Expression, and Transport Activity of Organic Anion Transporter 3
- PMID: 30761470
- PMCID: PMC6647857
- DOI: 10.1208/s12248-019-0303-4
Activation of Protein Kinase A Stimulates SUMOylation, Expression, and Transport Activity of Organic Anion Transporter 3
Abstract
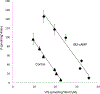
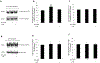
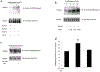
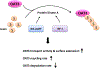
Organic anion transporter 3 (OAT3) plays a vital role in removing a broad variety of anionic drugs from kidney, thus avoiding their possible toxicity in the body. We earlier established that activation of protein kinase C (PKC) enhances OAT3 ubiquitination, which promotes OAT3 internalization from the cell plasma membrane to intracellular endosomes and consequent degradation. As a result, OAT3 expression and transport activity are reduced. In the current study, we discovered that protein kinase A (PKA) had an opposite effect to PKC on the regulation of OAT3. We showed that activation of PKA by Bt2-cAMP stimulated OAT3 transport activity, which was largely caused by an enhanced plasma membrane expression of the transporter, kinetically reflected as an augmented maximal transport velocity Vmax without notable alteration in substrate-binding affinity Km. Additionally, we showed that PKA activation accelerated the rate of OAT3 recycling from intracellular compartments to the plasma membrane and decelerated the rate of OAT3 degradation. We further showed that OAT3 is subjected to post-translational modification by SUMO-2 and SUMO-3 not by SUMO-1. PKA activation enhanced OAT3 SUMOylation, which was accompanied by a reduced OAT3 ubiquitination. Finally, insulin-like growth factor 1 significantly stimulated OAT3 transport activity and SUMOylation through PKA signaling pathway. In conclusion, this is the first demonstration that PKA stimulated OAT3 expression and transport activity by altering the trafficking kinetics of OAT3 possibly through the crosstalk between SUMOylation and ubiquitination. Our studies are consistent with a remote sensing and signaling model for transporters (Wu et al. in Mol Pharmacol. 79(5):795-805, 2011).
Keywords: SUMOylation; drug transport; organic anion transporter; protein kinase A; regulation.
Conflict of interest statement
Conflict of Interest
The authors have declared that there is no conflict of interest
Figures
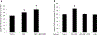
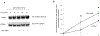
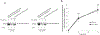
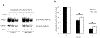
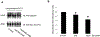
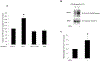
References
-
- Dantzler WH, Wright SH. The molecular and cellular physiology of basolateral organic anion transport in mammalian renal tubules. Biochim Biophys Acta. 2003;1618(2):185–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

